MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy
- PMID: 23326547
- PMCID: PMC3541228
- DOI: 10.1371/journal.pone.0053950
MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy
Abstract
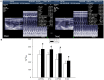
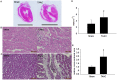
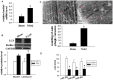
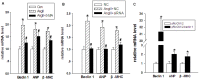
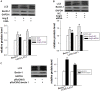
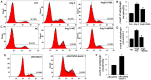
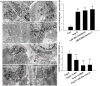
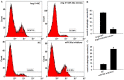
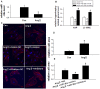
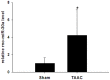
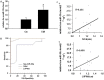
Dysregulated autophagy may lead to the development of disease. Role of autophagy and the diagnostic potential of microRNAs that regulate the autophagy in cardiac hypertrophy have not been evaluated. A rat model of cardiac hypertrophy was established using transverse abdominal aortic constriction (operation group). Cardiomyocyte autophagy was enhanced in rats from the operation group, compared with those in the sham operation group. Moreover, the operation group showed up-regulation of beclin-1 (an autophagy-related gene), and down-regulation of miR-30 in cardiac tissue. The effects of inhibition and over-expression of the beclin-1 gene on the expression of hypertrophy-related genes and on autophagy were assessed. Angiotensin II-induced myocardial hypertrophy was found to be mediated by over-expression of the beclin-1 gene. A dual luciferase reporter assay confirmed that beclin-1 was a target gene of miR-30a. miR-30a induced alterations in beclin-1 gene expression and autophagy in cardiomyocytes. Treatment of cardiomyocytes with miR-30a mimic attenuated the Angiotensin II-induced up-regulation of hypertrophy-related genes and decreased in the cardiomyocyte surface area. Conversely, treatment with miR-30a inhibitor enhanced the up-regulation of hypertrophy-related genes and increased the surface area of cardiomyocytes induced by Angiotensin II. In addition, circulating miR-30 was elevated in patients with left ventricular hypertrophy, and circulating miR-30 was positively associated with left ventricular wall thickness. Collectively, these above-mentioned results suggest that Angiotensin II induces down-regulation of miR-30 in cardiomyocytes, which in turn promotes myocardial hypertrophy through excessive autophagy. Circulating miR-30 may be an important marker for the diagnosis of left ventricular hypertrophy.
Conflict of interest statement
Figures

References
-
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, et al. (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress.Nat Med. 13: 619–624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

