H3N2v and other influenza epidemic risk based on age-specific estimates of sero-protection and contact network interactions
- PMID: 23326561
- PMCID: PMC3543419
- DOI: 10.1371/journal.pone.0054015
H3N2v and other influenza epidemic risk based on age-specific estimates of sero-protection and contact network interactions
Abstract
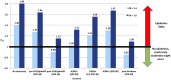
Cases of a novel swine-origin influenza A(H3N2) variant (H3N2v) have recently been identified in the US, primarily among children. We estimated potential epidemic attack rates (ARs) based on age-specific estimates of sero-susceptibility and social interactions. A contact network model previously established for the Greater Vancouver Area (GVA), Canada was used to estimate average epidemic (infection) ARs for the emerging H3N2v and comparator viruses (H1N1pdm09 and an extinguished H3N2 seasonal strain) based on typical influenza characteristics, basic reproduction number (R(0)), and effective contacts taking into account age-specific sero-protection rates (SPRs). SPRs were assessed in sera collected from the GVA in 2009 or earlier (pre-H1N1pdm09) and fall 2010 (post-H1N1pdm09, seasonal A/Brisbane/10/2007(H3N2), and H3N2v) by hemagglutination inhibition (HI) assay. SPR was assigned per convention based on proportion with HI antibody titre ≥40 (SPR40). Recognizing that the HI titre ≥40 was established as the 50%sero-protective threshold we also explored for ½SPR40, SPR80 and a blended gradient defined as: ¼SPR20, ½SPR40, ¾SPR80, SPR160. Base case analysis assumed R(0) = 1.40, but we also explored R(0) as high as 1.80. With R(0) = 1.40 and SPR40, simulated ARs were well aligned with field observations for H1N1pdm09 incidence (AR: 32%), sporadic detections without a third epidemic wave post-H1N1pdm09 (negligible AR<0.1%) as well as A/Brisbane/10/2007(H3N2) seasonal strain extinction and antigenic drift replacement (negligible AR<0.1%). Simulated AR for the novel swine-origin H3N2v was 6%, highest in children 6-11years (16%). However, with modification to SPR thresholds per above, H3N2v AR ≥20% became possible. At SPR40, H3N2v AR ≥10%, ≥15% or ≥30%, occur if R(0)≥1.48, ≥1.56 or ≥1.86, respectively. Based on conventional assumptions, the novel swine-origin H3N2v does not currently pose a substantial pandemic threat. If H3N2v epidemics do occur, overall community ARs are unlikely to exceed typical seasonal influenza experience. However risk assessment may change with time and depends crucially upon the validation of epidemiological features of influenza, notably the serologic correlate of protection and R(0).
Conflict of interest statement
Figures


References
-
- Cox RJ, Brokstad KA, Ogra P (2004) Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 59: 1–15. - PubMed
-
- Scholtissek C, Hinshaw VS, Olsen CV (1998) Influenza in pigs and their role as intermediate host. In: Nicholson KG, Webster KG, Hay A, editors. Textbook of Influenza. Oxford, UK: Blackwell Science. 137–145.
-
- Potter CW (1998) Chronicle of influenza pandemics. In: Nicholson KG, Webster KG, Hay A, editors. Textbook of Influenza. Oxford, UK: Blackwell Science. 3–18.
-
- Belshe RB (2005) The origins of pandemic influenza–lessons from the 1918 virus. N Engl J Med 353: 2209–2211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

