Age-associated molecular changes in the kidney in aged mice
- PMID: 23326623
- PMCID: PMC3544311
- DOI: 10.1155/2012/171383
Age-associated molecular changes in the kidney in aged mice
Abstract
Background: Aging is a multifactorial process characterized by a progressive decline in physiological function. Decreased kidney function is associated with cardiovascular disease and mortality. Therefore, increasing our insight into kidney aging by understanding the anatomic, physiologic, and pathologic changes of aging in the kidney is important to prevent disastrous outcomes in elderly people.
Methods: Male two-, 12-, and 24-month-old C57/BL6 mice were used in this study. We measured histological change, oxidative stress, and aging-related protein expression in the kidneys.
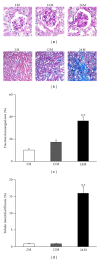
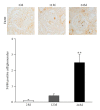
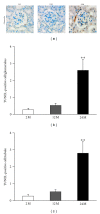
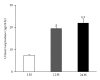
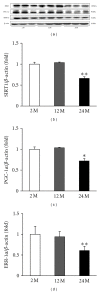
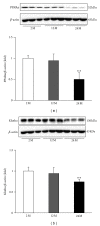
Results: Twenty-four-month-old mice displayed increased albuminuria. Creatinine clearance decreased with aging, although this was not statistically significant. There were increases in mesangial volume and tubulointerstitial fibrosis in 24-month-old mice. There were also increases in F4/80 expression and in apoptosis detected by TUNEL assay. Urine isoprostane excretion increased with aging and SOD1 and SOD2 were decreased in 24-month-old mice. Oxidative stress may be mediated by a decrease in Sirt1, PGC-1α, ERR-1α, and PPARα expression. Klotho expression also decreased.
Conclusions: Our results demonstrate that Sirt1 was decreased with aging and may relate to changed target molecules including PGC-1α/ERR-1α signaling and PPARα. Klotho can also induce oxidative stress. Pharmacologically targeting these signaling molecules may reduce the pathologic changes of aging in the kidney.
Figures


References
-
- Campbell KH, O’Hare AM. Kidney disease in the elderly: update on recent literature. Current Opinion in Nephrology and Hypertension. 2008;17(3):298–303. - PubMed
-
- O’Hare AM, Bertentha D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? Journal of the American Society of Nephrology. 2006;17(3):846–853. - PubMed
-
- Davies I, Fotheringham AP, Faragher BE. Age-associated changes in the kidney of the laboratory mouse. Age and Ageing. 1989;18(2):127–133. - PubMed
-
- Baylis C, Corman B. The aging kidney: insights from experimental studies. Journal of the American Society of Nephrology. 1998;9(4):699–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

