Clinical evaluation of BIOXTRA in relieving signs and symptoms of dry mouth after head and neck radiotherapy of cancer patients at Seyed-al-Shohada Hospital, Isfahan, Iran
- PMID: 23326802
- PMCID: PMC3544101
- DOI: 10.4103/2277-9175.102976
Clinical evaluation of BIOXTRA in relieving signs and symptoms of dry mouth after head and neck radiotherapy of cancer patients at Seyed-al-Shohada Hospital, Isfahan, Iran
Abstract
Background: Radiotherapy of head and neck cancers causes acute and chronic xerostomia and acute mucositis. Xerostomia increases risk of radiation caries and affects on oral comfort, fit of prostheses, speech, swallowing, and the growth of caries-producing organisms. Salivary flow rate can be measured by asking patients some questions. There are different types of commercial synthetic saliva such as BIOXTRA, but until now, no one can effectively relieve xerostomia. We tried to design a clinical research on BIOXTRA efficacy for treating xerostomia.
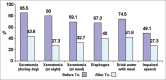
Materials and methods: In this research, 58 patients with head and neck cancer (except salivary gland cancers) treated in Seyed-al-Shohada Hospital. The patients received at least 40-50 GY; and after 2 months of compilation treatment, they were evaluated by asking about having xerostomia. Before and after treatment with the BIOXTRA, the PH of the oral cavity, candida albicans, and lactobacillus counts measured and documented in laboratory. We used BIOXTRA for 2 weeks, 3 times daily, and then re-evaluated patients with some questions.
Results: The counts of candida albicans and lactobacilli statistically significant decreased.
Conclusion: Xerostomia for most patients improved clinically during the day and night while PH of the oral cavity increased.
Keywords: BIOXTRA; radiotherapy; xerostomia.
Conflict of interest statement
Figures
Similar articles
-
Efficacy of the BioXtra dry mouth care system in the treatment of radiotherapy-induced xerostomia.Support Care Cancer. 2007 Dec;15(12):1429-36. doi: 10.1007/s00520-006-0210-y. Epub 2007 Jan 18. Support Care Cancer. 2007. PMID: 17235501
-
Clinical evaluation of a commercially available oral moisturizer in relieving signs and symptoms of xerostomia in postirradiation head and neck cancer patients and patients with Sjögren's syndrome.J Otolaryngol. 2000 Feb;29(1):28-34. J Otolaryngol. 2000. PMID: 10709169
-
Oral pilocarpine for radiation-induced xerostomia: integrated efficacy and safety results from two prospective randomized clinical trials.Int J Radiat Oncol Biol Phys. 1995 Feb 1;31(3):661-9. doi: 10.1016/0360-3016(94)00361-N. Int J Radiat Oncol Biol Phys. 1995. PMID: 7852133 Clinical Trial.
-
Radiation-induced xerostomia: pathophysiology, clinical course and supportive treatment.Support Care Cancer. 1997 Jul;5(4):281-8. doi: 10.1007/s005200050075. Support Care Cancer. 1997. PMID: 9257424 Review.
-
Xerostomia: diagnosis and management.Oncology (Williston Park). 1996 Mar;10(3 Suppl):7-11. Oncology (Williston Park). 1996. PMID: 8723427 Review.
Cited by
-
Influence of irradiated dentin, biofilm and different artificial saliva formulations on root dentin demineralization.Heliyon. 2024 Aug 14;10(16):e36334. doi: 10.1016/j.heliyon.2024.e36334. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39247326 Free PMC article.
-
Efficacy of gel-based artificial saliva on Candida colonization and saliva properties in xerostomic post-radiotherapy head and neck cancer patients: a randomized controlled trial.Clin Oral Investig. 2021 Apr;25(4):1815-1827. doi: 10.1007/s00784-020-03484-1. Epub 2020 Aug 10. Clin Oral Investig. 2021. PMID: 32779011 Free PMC article. Clinical Trial.
-
Current developments and opportunities of pluripotent stem cells-based therapies for salivary gland hypofunction.Front Cell Dev Biol. 2024 Jan 19;12:1346996. doi: 10.3389/fcell.2024.1346996. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38313227 Free PMC article. Review.
-
Efficacy of Artificial Salivary Substitutes in Treatment of Xerostomia: A Systematic Review.J Pharm Bioallied Sci. 2019 Feb;11(Suppl 1):S1-S12. doi: 10.4103/jpbs.JPBS_220_18. J Pharm Bioallied Sci. 2019. PMID: 30923424 Free PMC article. Review.
-
Saliva substitute mouthwash in nasopharyngeal cancer survivors with xerostomia: a randomized controlled trial.Clin Oral Investig. 2021 May;25(5):3105-3115. doi: 10.1007/s00784-020-03634-5. Epub 2020 Nov 11. Clin Oral Investig. 2021. PMID: 33175253 Free PMC article. Clinical Trial.
References
-
- Tschiesner U, Rogers SN, Harréus U, Berghaus A, Cieza A. Content comparison of quality of life questionnaires used in head and neck cancer based on the international classification of functioning, disability and health: A systematic review. Eur Arch Otorhinolaryngol. 2008;265:627–37. - PubMed
-
- Perez CA. Brady's. Principles and practice of radiation oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins Company; 2008. pp. 2–137.
-
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. - PubMed
-
- Ghezzi EM, Lange LA, Ship JA. Determination of variation of stimulated salivary flow rates. J Dent Res. 2000;79:1874–8. - PubMed
-
- Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992;71:1363–9. - PubMed