Reprogrammed cells for disease modeling and regenerative medicine
- PMID: 23327523
- PMCID: PMC3629705
- DOI: 10.1146/annurev-med-050311-163324
Reprogrammed cells for disease modeling and regenerative medicine
Abstract
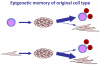
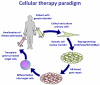
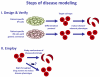
The conversion of somatic cells into pluripotent cells is transforming the way diseases are researched and treated. Induced pluripotent stem (iPS) cells' promise may soon be realized in the field of hematology, as hematopoietic stem cell transplants are already commonplace in clinics around the world. We provide a current comparison between induced pluripotent and embryonic stem cells, describe progress toward modeling hematological disorders using iPS cells, and illustrate the hurdles that must be overcome before iPS cell therapies will be available in clinics.
Figures
References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–47. - PubMed
-
- Gottweis H, Prainsack B. Emotion in political discourse: contrasting approaches to stem cell governance in the USA, UK, Israel and Germany. Regenerative Medicine. 2006;1:823–29. - PubMed
-
- Takahashi K,, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
-
- Davis RL, Weintraub H, Lassar AB. EXPRESSION OF A SINGLE TRANSFECTED CDNA CONVERTS FIBROBLASTS TO MYOBLASTS. Cell. 1987;51:987–1000. - PubMed
-
- Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–U2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

