Integrated proteomic analysis of major isoaspartyl-containing proteins in the urine of wild type and protein L-isoaspartate O-methyltransferase-deficient mice
- PMID: 23327623
- PMCID: PMC3599293
- DOI: 10.1021/ac303428h
Integrated proteomic analysis of major isoaspartyl-containing proteins in the urine of wild type and protein L-isoaspartate O-methyltransferase-deficient mice
Abstract
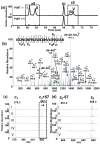
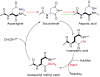
The formation of isoaspartyl residues (isoAsp or isoD) via either aspartyl isomerization or asparaginyl deamidation alters protein structure and potentially biological function. This is a spontaneous and nonenzymatic process, ubiquitous both in vivo and in nonbiological systems, such as in protein pharmaceuticals. In almost all organisms, protein L-isoaspartate O-methyltransferase (PIMT, EC2.1.1.77) recognizes and initiates the conversion of isoAsp back to aspartic acid. Additionally, alternative proteolytic and excretion pathways to metabolize isoaspartyl-containing proteins have been proposed but not fully explored, largely due to the analytical challenges for detecting isoAsp. We report here the relative quantitation and site profiling of isoAsp in urinary proteins from wild type and PIMT-deficient mice, representing products from excretion pathways. First, using a biochemical approach, we found that the total isoaspartyl level of proteins in urine of PIMT-deficient male mice was elevated. Subsequently, the major isoaspartyl protein species in urine from these mice were identified as major urinary proteins (MUPs) by shotgun proteomics. To enhance the sensitivity of isoAsp detection, a targeted proteomic approach using electron transfer dissociation-selected reaction monitoring (ETD-SRM) was developed to investigate isoAsp sites in MUPs. A total of 38 putative isoAsp modification sites in MUPs were investigated, with five derived from the deamidation of asparagine that were confirmed to contribute to the elevated isoAsp levels. Our findings lend experimental evidence for the hypothesized excretion pathway for isoAsp proteins. Additionally, the developed method opens up the possibility to explore processing mechanisms of isoaspartyl proteins at the molecular level, such as the fate of protein pharmaceuticals in circulation.
Figures


References
-
- Geiger T, Clarke S. J Biol Chem. 1987;262:785–94. - PubMed
-
- Shimizu T, Matsuoka Y, Shirasawa T. Biol Pharm Bull. 2005;28:1590–6. DOI: JST.JSTAGE/bpb/28.1590 [pii] - PubMed
-
- Clarke S. Ageing Res Rev. 2003;2:263–85. DOI: JS1568163703000114 [pii] - PubMed
-
- Furuchi T, Homma H. Yakugaku Zasshi. 2007;127:1927–36. DOI: JST.JSTAGE/yakushi/127.1927 [pii] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

