[Detection of EGFR gene mutations in 100 non-small cell lung cancer clinical samples by a real-time polymerase chain reaction method using amplification refractory mutation system specific primers and Taqman fluorescence probes]
- PMID: 23327870
- PMCID: PMC6000453
- DOI: 10.3779/j.issn.1009-3419.2013.01.05
[Detection of EGFR gene mutations in 100 non-small cell lung cancer clinical samples by a real-time polymerase chain reaction method using amplification refractory mutation system specific primers and Taqman fluorescence probes]
Abstract
Background: Epidermal growth factor receptor (EGFR) gene mutation is the most important predictor of the efficiency of EGFR-tyrosine kinase inhibitors in the treatment of non-small cell lung cancer (NSCLC). The detection of EGFR gene mutations can guide individual therapies for NSCLC. Numerous methods are used to detect EGFR gene mutation and each method has different features. This study aims to establish a real-time polymerase chain reaction (PCR) method for the detection of EGFR gene mutations using amplification refractory mutation system (ARMS) specific primers and Taqman fluorescence probes.
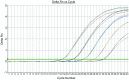
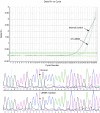
Methods: ARMS specific primers for the two EGFR gene mutations (E746_A750 and L858R) and Taqman fluorescence probes for the detection of the target sequence were carefully designed by the Primer Premier 5.0 software. Then, using the recombinants containing E746_A750 and L858R mutations as the study objects, we further analyzed the sensitivity and lower limit of this method, and then determined the cutoff ΔCt value to evaluate specific or non-specific amplification. A total of 100 clinical samples were collected and used to detect the EGFR gene mutations using this method.
Results: The lower limit of this method for the detection of EGFR gene mutation was 10 copies if no interference of wild-type EGFR gene or background DNA existed. Regarding the method sensitivity, the detection resolution was as high as 1% and 0.1%-0.5% in the background of 500 and 5,000 copies/μL wild-type EGFR gene, respectively. Regarding the method specificity, non-specific amplifications were found when it was used to detect 21 L858R mutations in leukocyte DNA samples from healthy volunteers. However, the minimal ΔCt value was 14.48. Non-specific amplifications were not found when detecting 19 Del mutations. Among the 100 clinical samples, 39 mutations were detected (19 Del and 21 L858R were 21 and 18, respectively) using this method. The total mutation rate was 39.0%.
Conclusions: The proposed ARMS-TaqMan real-time PCR method for the detection of 19 Del and 21 L858R mutations in EGFR gene was rapid, simple, sensitive, specific, and applicable in the clinical setting.
背景与目的: 表皮生长因子受体(epidermal growth factor receptor, EGFR)基因突变是决定表皮生长因子受体酪氨酸激酶抑制剂(EGFR tyrosine kinase inhibitor, EGFR-TKI)疗效最重要的预测因子,对EGFR基因突变进行检测,对指导患者个体化治疗具有重要意义。EGFR基因突变检测方法有很多,每种方法各有优缺点,本研究拟采用扩增阻滞突变系统(amplification refractory mutation system, ARMS)技术与Taqman探针相结合的方法,建立一种能快速、敏感及特异检测非小细胞肺癌EGFR基因突变的方法。
方法: 首先,应用Primer Premier 5.0软件在EGFR基因E746_A750和L858R处设计ARMS引物及Taqman水解探针。然后,以包含E746_A750缺失和L858R点突变的质粒为研究对象,进一步分析所建立方法的灵敏度、敏感性以及特异性。最后,用所建立的ARMS-Taqman法检测100例非小细胞肺癌(non-small cell lung cancer, NSCLC)临床标本。
结果: 在无背景DNA干扰的情况下,ARMS-Taqman法检测灵敏度可达10 copies。对于检测敏感性,在500 copies/μL野生型基因背景下,其敏感性达1%;在5, 000 copies/μl野生型基因背景下,其敏感性高达0.1%-0.5%。对于检测特异性,以正常人白细胞DNA为研究对象,21 L858R突变存在一定程度的非特异性扩增,但其最小ΔCt高达14.89,而19 Del未见非特异性扩增。对100份临床标本进行检测,19 Del 21例,21 L858R 18例,总突变率为39.0%。
结论: 我们所构建的ARMS-Taqman法是一种快速、简便以及具有较高灵敏度和特异性的EGFR基因突变检测方法,值得在临床上进一步推广和验证。
Figures






Similar articles
-
A simple and sensitive method for detecting major mutations within the tyrosine kinase domain of the epidermal growth factor receptor gene in non-small-cell lung carcinoma.Diagn Mol Pathol. 2006 Jun;15(2):101-8. doi: 10.1097/00019606-200606000-00007. Diagn Mol Pathol. 2006. PMID: 16778591
-
A comparison of ARMS and mutation specific IHC for common activating EGFR mutations analysis in small biopsy and cytology specimens of advanced non small cell lung cancer.Int J Clin Exp Pathol. 2014 Jun 15;7(7):4310-6. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25120814 Free PMC article.
-
A novel, rapid point-of-care test for lung cancer patients to detect epidermal growth factor receptor gene mutations by using real-time droplet-PCR and fresh liquid cytology specimens.Oncol Rep. 2017 Feb;37(2):1020-1026. doi: 10.3892/or.2016.5287. Epub 2016 Dec 2. Oncol Rep. 2017. PMID: 27922678
-
Profile of the therascreen® EGFR RGQ PCR kit as a companion diagnostic for gefitinib in non-small cell lung cancer.Expert Rev Mol Diagn. 2016 Dec;16(12):1251-1257. doi: 10.1080/14737159.2016.1248414. Epub 2016 Oct 27. Expert Rev Mol Diagn. 2016. PMID: 27737606 Review.
-
A profile on cobas® EGFR Mutation Test v2 as companion diagnostic for first-line treatment of patients with non-small cell lung cancer.Expert Rev Mol Diagn. 2020 Jun;20(6):575-582. doi: 10.1080/14737159.2020.1724094. Epub 2020 Feb 20. Expert Rev Mol Diagn. 2020. PMID: 32011193 Review.
Cited by
-
Prediction of the efficacy and clinical prognosis of first-line EGFR-tyrosine kinase inhibitors in non-small cell lung cancer patients based on ΔCt values derived from the super-amplification refractory mutation system (ARMS): a real-world retrospective study.J Thorac Dis. 2025 Jun 30;17(6):3897-3911. doi: 10.21037/jtd-2025-97. Epub 2025 Jun 25. J Thorac Dis. 2025. PMID: 40688317 Free PMC article.
-
LDA-SVM-based EGFR mutation model for NSCLC brain metastases: an observational study.Medicine (Baltimore). 2015 Feb;94(5):e375. doi: 10.1097/MD.0000000000000375. Medicine (Baltimore). 2015. PMID: 25654374 Free PMC article.
References
-
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. - DOI - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

