MASL1 induces erythroid differentiation in human erythropoietin-dependent CD34+ cells through the Raf/MEK/ERK pathway
- PMID: 23327923
- PMCID: PMC3630834
- DOI: 10.1182/blood-2011-10-385252
MASL1 induces erythroid differentiation in human erythropoietin-dependent CD34+ cells through the Raf/MEK/ERK pathway
Abstract
Human erythropoiesis is a dynamic and complex multistep process involving differentiation of early erythroid progenitors into enucleated RBCs. The mechanisms underlying erythropoiesis still remain incompletely understood. We previously demonstrated that erythropoietin-stimulated clone-1, which is selectively expressed in normal human erythroid-lineage cells, shares 99.5% identity with malignant fibrous histiocytoma-amplified sequences with leucine-rich tandem repeats 1 (MASL1). In this study, we hypothesized that the MASL1 gene plays a role in erythroid differentiation, and used a human erythroid cell culture system to explore this concept. MASL1 mRNA and protein expression levels were significantly increased during the erythroid differentiation of CD34(+) cells following erythropoietin (EPO) treatment. Conversely, MASL1 knockdown reduced erythroid differentiation in EPO-treated CD34(+) cells. In addition, MASL1 knockdown interrupted the Raf/MEK/ERK signaling pathway in CD34(+) cells. MASL1 mutant-transfected CD34(+) cells also showed decreased erythroid differentiation. Furthermore, inhibition of the SH3 domain of Son of Sevenless, which is an upstream adapter protein in EPO-induced erythroid differentiation, also reduced MASL1 expression and phosphorylation of Raf/MEK/ERK kinases that consequently reduced erythroid differentiation of EPO-induced CD34(+) cells. Importantly, we also demonstrated that MASL1 interacts physically with Raf1. Taken together, our data provide novel insights into MASL1 regulation of erythropoiesis through the Raf/MEK/ERK pathway.
Figures
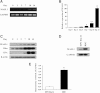



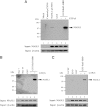
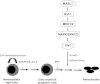
Comment in
-
Another path to ERK activation.Blood. 2013 Apr 18;121(16):3064-5. doi: 10.1182/blood-2013-02-480459. Blood. 2013. PMID: 23599259 No abstract available.
Similar articles
-
Another path to ERK activation.Blood. 2013 Apr 18;121(16):3064-5. doi: 10.1182/blood-2013-02-480459. Blood. 2013. PMID: 23599259 No abstract available.
-
Constitutive activation of the MEK/ERK pathway mediates all effects of oncogenic H-ras expression in primary erythroid progenitors.Blood. 2004 Sep 15;104(6):1679-87. doi: 10.1182/blood-2004-04-1362. Epub 2004 May 27. Blood. 2004. PMID: 15166036
-
Activation of phosphatidylinositol 3-kinase is important for erythropoietin-induced erythropoiesis from CD34(+) hematopoietic progenitor cells.Exp Hematol. 2002 Sep;30(9):990-1000. doi: 10.1016/s0301-472x(02)00868-8. Exp Hematol. 2002. PMID: 12225790
-
MASL1: a neglected ROCO protein.Biochem Soc Trans. 2012 Oct;40(5):1090-4. doi: 10.1042/BST20120127. Biochem Soc Trans. 2012. PMID: 22988871 Review.
-
The role of tyrosine phosphorylation in proliferation and maturation of erythroid progenitor cells--signals emanating from the erythropoietin receptor.Eur J Biochem. 1997 Nov 1;249(3):637-47. doi: 10.1111/j.1432-1033.1997.t01-1-00637.x. Eur J Biochem. 1997. PMID: 9395308 Review.
Cited by
-
Governing roles for Trib3 pseudokinase during stress erythropoiesis.Exp Hematol. 2017 May;49:48-55.e5. doi: 10.1016/j.exphem.2016.12.010. Epub 2017 Jan 4. Exp Hematol. 2017. PMID: 28062363 Free PMC article.
-
Erythropoietin regulation of red blood cell production: from bench to bedside and back.F1000Res. 2020 Sep 18;9:F1000 Faculty Rev-1153. doi: 10.12688/f1000research.26648.1. eCollection 2020. F1000Res. 2020. PMID: 32983414 Free PMC article. Review.
-
Single-cell transcriptome sequencing reveals the mechanism of Realgar improvement on erythropoiesis in mice with myelodysplastic syndrome.Cancer Cell Int. 2025 Apr 8;25(1):135. doi: 10.1186/s12935-025-03768-0. Cancer Cell Int. 2025. PMID: 40200265 Free PMC article.
-
PIEZO1 activation delays erythroid differentiation of normal and hereditary xerocytosis-derived human progenitor cells.Haematologica. 2020 Mar;105(3):610-622. doi: 10.3324/haematol.2019.218503. Epub 2019 Aug 14. Haematologica. 2020. PMID: 31413092 Free PMC article.
-
RHEX, a novel regulator of human erythroid progenitor cell expansion and erythroblast development.J Exp Med. 2014 Aug 25;211(9):1715-22. doi: 10.1084/jem.20130624. Epub 2014 Aug 4. J Exp Med. 2014. PMID: 25092874 Free PMC article.
References
-
- Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000;1(1):57–64. - PubMed
-
- Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002;21(21):3368–3376. - PubMed
-
- Zhang J, Lodish HF. Constitutive activation of the MEK/ERK pathway mediates all effects of oncogenic H-ras expression in primary erythroid progenitors. Blood. 2004;104(6):1679–1687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

