Genomic imbalance of HMMR/RHAMM regulates the sensitivity and response of malignant peripheral nerve sheath tumour cells to aurora kinase inhibition
- PMID: 23328114
- PMCID: PMC3702209
- DOI: 10.18632/oncotarget.793
Genomic imbalance of HMMR/RHAMM regulates the sensitivity and response of malignant peripheral nerve sheath tumour cells to aurora kinase inhibition
Abstract
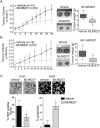
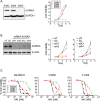
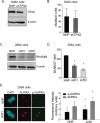
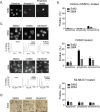
Malignant peripheral nerve sheath tumours (MPNST) are rare, hereditary cancers associated with neurofibromatosis type I. MPNSTs lack effective treatment options as they often resist chemotherapies and have high rates of disease recurrence. Aurora kinase A (AURKA) is an emerging target in cancer and an aurora kinase inhibitor (AKI), termed MLN8237, shows promise against MPNST cell lines in vitro and in vivo. Here, we test MLN8237 against two primary human MPNST grown in vivo as xenotransplants and find that treatment results in tumour cells exiting the cell cycle and undergoing endoreduplication, which cumulates in stabilized disease. Targeted therapies can often fail in the clinic due to insufficient knowledge about factors that determine tumour susceptibilities, so we turned to three MPNST cell-lines to further study and modulate the cellular responses to AKI. We find that the sensitivity of cell-lines with amplification of AURKA depends upon the activity of the kinase, which correlates with the expression of the regulatory gene products TPX2 and HMMR/RHAMM. Silencing of HMMR/RHAMM, but not TPX2, augments AURKA activity and sensitizes MPNST cells to AKI. Furthermore, we find that AURKA activity is critical to the propagation and self-renewal of sphere-enriched MPNST cancer stem-like cells. AKI treatment significantly reduces the formation of spheroids, attenuates the self-renewal of spheroid forming cells, and promotes their differentiation. Moreover, silencing of HMMR/RHAMM is sufficient to endow MPNST cells with an ability to form and maintain sphere culture. Collectively, our data indicate that AURKA is a rationale therapeutic target for MPNST and tumour cell responses to AKI, which include differentiation, are modulated by the abundance of HMMR/RHAMM.
Figures

References
-
- Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12(1):1–11. - PubMed
-
- Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10(5):508–515. - PubMed
-
- Woodruff JM. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am J Med Genet. 1999;89(1):23–30. - PubMed
-
- Hagel C, Zils U, Peiper M, Kluwe L, Gotthard S, Friedrich RE, Zurakowski D, von Deimling A, Mautner VF. Histopathology and clinical outcome of NF1-associated vs. sporadic malignant peripheral nerve sheath tumors. J Neurooncol. 2007;82(2):187–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

