NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers
- PMID: 23328480
- PMCID: PMC3954609
- DOI: 10.1016/j.ccr.2012.12.001
NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers
Abstract
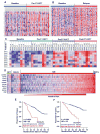
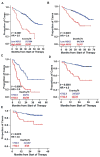
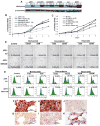
Using sequential gene expression profiling (GEP) samples, we defined a major functional group related to drug resistance that contains chromosomal instability (CIN) genes. One CIN gene in particular, NEK2, was highly correlated with drug resistance, rapid relapse, and poor outcome in multiple cancers. Overexpressing NEK2 in cancer cells resulted in enhanced CIN, cell proliferation and drug resistance, while targeting NEK2 by NEK2 shRNA overcame cancer cell drug resistance and induced apoptosis in vitro and in a xenograft myeloma mouse model. High expression of NEK2 induced drug resistance mainly through activation of the efflux pumps. Thus, NEK2 represents a strong predictor for drug resistance and poor prognosis in cancer and could be an important target for cancer therapy.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict – of – interest disclosure.
Figures



References
-
- Avet-Loiseau H, Facon T, Grosbois B, Magrangeas F, Rapp MJ, Harousseau JL, Minvielle S, Bataille R. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation. Blood. 2002;99:2185–2191. - PubMed
-
- Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, Fassas A, Zangari M, Hollmig K, Pineda-Roman M, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. - PubMed
-
- Bienz M. The subcellular destinations of APC proteins. Nature Reviews Molecular Cell Biology. 2002;3:328–338. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

