Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation
- PMID: 23328522
- PMCID: PMC3603595
- DOI: 10.1164/rccm.201207-1294OC
Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation
Abstract
Rationale: The role of reactive oxygen species (ROS) signaling in the O(2) sensing mechanism underlying acute hypoxic pulmonary vasoconstriction (HPV) has been controversial. Although mitochondria are important sources of ROS, studies using chemical inhibitors have yielded conflicting results, whereas cellular models using genetic suppression have precluded in vivo confirmation. Hence, genetic animal models are required to test mechanistic hypotheses.
Objectives: We tested whether mitochondrial Complex III is required for the ROS signaling and vasoconstriction responses to acute hypoxia in pulmonary arteries (PA).
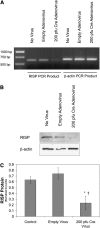
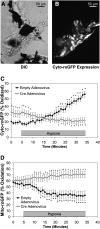
Methods: A mouse permitting Cre-mediated conditional deletion of the Rieske iron-sulfur protein (RISP) of Complex III was generated. Adenoviral Cre recombinase was used to delete RISP from isolated PA vessels or smooth muscle cells (PASMC).
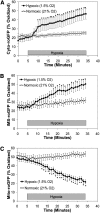
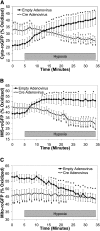
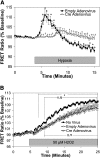
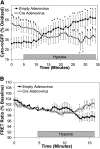
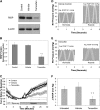
Measurements and main results: In PASMC, RISP depletion abolished hypoxia-induced increases in ROS signaling in the mitochondrial intermembrane space and cytosol, and it abrogated hypoxia-induced increases in [Ca(2+)](i). In isolated PA vessels, RISP depletion abolished hypoxia-induced ROS signaling in the cytosol. Breeding the RISP mice with transgenic mice expressing tamoxifen-activated Cre in smooth muscle permitted the depletion of RISP in PASMC in vivo. Precision-cut lung slices from those mice revealed that RISP depletion abolished hypoxia-induced increases in [Ca(2+)](i) of the PA. In vivo RISP depletion in smooth muscle attenuated the acute hypoxia-induced increase in right ventricular systolic pressure in anesthetized mice.
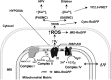
Conclusions: Acute hypoxia induces superoxide release from Complex III of smooth muscle cells. These oxidant signals diffuse into the cytosol and trigger increases in [Ca(2+)](i) that cause acute hypoxic pulmonary vasoconstriction.
Figures
Comment in
-
Mitochondria in hypoxic pulmonary vasoconstriction: potential importance of compartmentalized reactive oxygen species signaling.Am J Respir Crit Care Med. 2013 Feb 15;187(4):338-40. doi: 10.1164/rccm.201301-0037ED. Am J Respir Crit Care Med. 2013. PMID: 23418325 Free PMC article. No abstract available.
References
-
- Rounds S, McMurtry IF. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res 1981;48:393–400 - PubMed
-
- McMurtry IF. Angiotensin is not required for hypoxic constriction in salt solution-perfused rat lungs. J Appl Physiol 1984;56:375–380 - PubMed
-
- Grimminger F, Weissmann N, Spriestersbach R, Becker E, Rosseau S, Seeger W. Effects of NADPH oxidase inhibitors on hypoxic vasoconstriction in buffer-perfused rabbit lungs. Am J Physiol 1995;268:L747–L752 - PubMed
-
- Weissmann N, Grimminger F, Voswinckel R, Conzen J, Seeger W. Nitro blue tetrazolium inhibits but does not mimic hypoxic vasoconstriction in isolated rabbit lungs. Am J Physiol 1998;274:L721–L727 - PubMed
-
- Weissmann N, Grimminger F, Walmrath D, Seeger W. Hypoxic vasoconstriction in buffer-perfused rabbit lungs. Respir Physiol 1995;100:159–169 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

