Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth
- PMID: 23328584
- PMCID: PMC3602275
- DOI: 10.1158/0008-5472.CAN-12-2474
Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth
Abstract
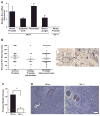
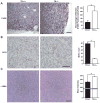
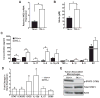
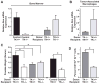
Ron receptor kinase (MST1R) is important in promoting epithelial tumorigenesis, but the potential contributions of its specific expression in stromal cells have not been examined. Herein, we show that the Ron receptor is expressed in mouse and human stromal cells of the prostate tumor microenvironment. To test the significance of stromal Ron expression, prostate cancer cells were orthotopically implanted into the prostates of either wild-type or Ron tyrosine kinase deficient (TK(-/-); Mst1r(-/-)) hosts. In TK(-/-) hosts, prostate cancer cell growth was significantly reduced as compared with tumor growth in TK(+/+) hosts. Prostate tumors in TK(-/-) hosts exhibited an increase in tumor cell apoptosis, macrophage infiltration and altered cytokine expression. Reciprocal bone marrow transplantation studies and myeloid cell-specific ablation of Ron showed that loss of Ron in myeloid cells is sufficient to inhibit prostate cancer cell growth. Interestingly, depletion of CD8(+) T cells, but not CD4(+) T cells, was able to restore prostate tumor growth in hosts devoid of myeloid-specific Ron expression. These studies show a critical role for the Ron receptor in the tumor microenvironment, whereby Ron loss in tumor-associated macrophages inhibits prostate cancer cell growth, at least in part, by derepressing the activity of CD8(+) T cells.
Conflict of interest statement
Figures

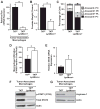
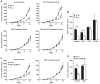
References
-
- Condon MS. The role of the stromal microenvironment in prostate cancer. Semin Cancer Biol. 2005;15:132–7. - PubMed
-
- Grossfeld GDSWH, Tlsty TD, Cunha GR. The role of stroma in prostatic carcinogenesis. Endocrine-Related Cancer. 1998;5:253–70.
-
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

