Mast cells rescue implantation defects caused by c-kit deficiency
- PMID: 23328669
- PMCID: PMC3564001
- DOI: 10.1038/cddis.2012.214
Mast cells rescue implantation defects caused by c-kit deficiency
Abstract
Various physiologically relevant processes are regulated by the interaction of the receptor tyrosine kinase (c-Kit) and its ligand stem cell factor (SCF), with SCF known to be the most important growth factor for mast cells (MCs). In spite of their traditional role in allergic disorders and innate immunity, MCs have lately emerged as versatile modulators of a variety of physiologic and pathologic processes. Here we show that MCs are critical for pregnancy success. Uterine MCs presented a unique phenotype, accumulated during receptivity and expanded upon pregnancy establishment. Kit(W-sh/W-sh) mice, whose MC deficiency is based on restricted c-Kit gene expression, exhibited severely impaired implantation, which could be completely rescued by systemic or local transfer of wild-type bone marrow-derived MCs. Transferred wild-type MCs favored normal implantation, induced optimal spiral artery remodeling and promoted the expression of MC proteases, transforming growth factor-β and connective tissue growth factor. MCs contributed to trophoblast survival, placentation and fetal growth through secretion of the glycan-binding protein galectin-1. Our data unveil unrecognized roles for MCs at the fetomaternal interface with critical implications in reproductive medicine.
Figures
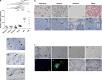


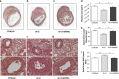
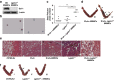
References
-
- Dastych J, Metcalfe DD. Stem cell factor induces mast cell adhesion to fibronectin. J Immunol. 1994;152:213–219. - PubMed
-
- Ohta H, Yomogida K, Dohmae K, Nishimune Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–2131. - PubMed
-
- Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod. 2006;75:421–433. - PubMed
-
- Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

