Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson's disease
- PMID: 23328674
- PMCID: PMC3563993
- DOI: 10.1038/cddis.2012.205
Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson's disease
Abstract
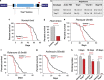
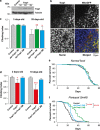
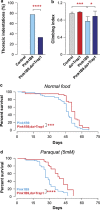
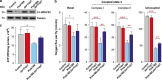
Mitochondrial dysfunction caused by protein aggregation has been shown to have an important role in neurological diseases, such as Parkinson's disease (PD). Mitochondria have evolved at least two levels of defence mechanisms that ensure their integrity and the viability of their host cell. First, molecular quality control, through the upregulation of mitochondrial chaperones and proteases, guarantees the clearance of damaged proteins. Second, organellar quality control ensures the clearance of defective mitochondria through their selective autophagy. Studies in Drosophila have highlighted mitochondrial dysfunction linked with the loss of the PTEN-induced putative kinase 1 (PINK1) as a mechanism of PD pathogenesis. The mitochondrial chaperone TNF receptor-associated protein 1 (TRAP1) was recently reported to be a cellular substrate for the PINK1 kinase. Here, we characterise Drosophila Trap1 null mutants and describe the genetic analysis of Trap1 function with Pink1 and parkin. We show that loss of Trap1 results in a decrease in mitochondrial function and increased sensitivity to stress, and that its upregulation in neurons of Pink1 mutant rescues mitochondrial impairment. Additionally, the expression of Trap1 was able to partially rescue mitochondrial impairment in parkin mutant flies; and conversely, expression of parkin rescued mitochondrial impairment in Trap1 mutants. We conclude that Trap1 works downstream of Pink1 and in parallel with parkin in Drosophila, and that enhancing its function may ameliorate mitochondrial dysfunction and rescue neurodegeneration in PD.
Figures

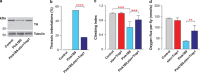
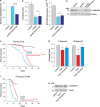
References
-
- Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J Cell Sci. 2010;123:3849–3855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

