Deletion of Nrf2 impairs functional recovery, reduces clearance of myelin debris and decreases axonal remyelination after peripheral nerve injury
- PMID: 23328769
- PMCID: PMC3628945
- DOI: 10.1016/j.nbd.2013.01.003
Deletion of Nrf2 impairs functional recovery, reduces clearance of myelin debris and decreases axonal remyelination after peripheral nerve injury
Abstract
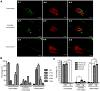
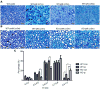
Oxidative stress is generated in several peripheral nerve injury models. In response to oxidative stress, the transcription factor Nrf2 is activated to induce expression of antioxidant responsive element (ARE) genes. The role of Nrf2 in peripheral nerve injury has not been studied to date. In this study, we used a sciatic nerve crush model to examine how deletion of Nrf2 affects peripheral nerve degeneration and regeneration. Our study demonstrated that functional recovery in the Nrf2(-/-) mice were impaired compared to the wild type mice after sciatic nerve crush. Larger myelin debris were present in the distal nerve stump of the Nrf2(-/-) mice than in the wild type mice. The presence of larger myelin debris in the Nrf2(-/-) mice coincides with less macrophages accumulation in the distal nerve stump. Less accumulation of macrophages may have contributed to slower clearance of myelin and thus resulted in the presence of larger myelin debris. Meanwhile, axonal regeneration is comparatively lower in the Nrf2(-/-) mice than in the wild type mice. Even after 3months post the injury, more thinly myelinated axon fibers were present in the Nrf2(-/-) mice than in the wild type mice. Taken collectively, these data support the concept of therapeutic intervention with Nrf2 activators following nerve injury.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



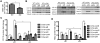

Similar articles
-
Deletion of SIRPα (signal regulatory protein-α) promotes phagocytic clearance of myelin debris in Wallerian degeneration, axon regeneration, and recovery from nerve injury.J Neuroinflammation. 2019 Dec 28;16(1):277. doi: 10.1186/s12974-019-1679-x. J Neuroinflammation. 2019. PMID: 31883525 Free PMC article.
-
Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination.Brain. 2013 Jul;136(Pt 7):2279-97. doi: 10.1093/brain/awt148. Brain. 2013. PMID: 23801741 Free PMC article.
-
Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury.J Neurosci. 2015 Dec 16;35(50):16431-42. doi: 10.1523/JNEUROSCI.2119-15.2015. J Neurosci. 2015. PMID: 26674868 Free PMC article.
-
Prevention of Axonal Degeneration by Perineurium Injection of Mitochondria in a Sciatic Nerve Crush Injury Model.Neurosurgery. 2017 Mar 1;80(3):475-488. doi: 10.1093/neuros/nyw090. Neurosurgery. 2017. PMID: 28362972
-
Transection and Crush Models of Nerve Injury to Measure Repair and Remyelination in Peripheral Nerve.Methods Mol Biol. 2018;1791:251-262. doi: 10.1007/978-1-4939-7862-5_20. Methods Mol Biol. 2018. PMID: 30006716
Cited by
-
Protective effects of sulforaphane in experimental vascular cognitive impairment: Contribution of the Nrf2 pathway.J Cereb Blood Flow Metab. 2019 Feb;39(2):352-366. doi: 10.1177/0271678X18764083. Epub 2018 Mar 13. J Cereb Blood Flow Metab. 2019. PMID: 29533123 Free PMC article.
-
Gastrodin promotes the regeneration of peripheral nerves by regulating miR-497/BDNF axis.BMC Complement Med Ther. 2022 Feb 18;22(1):45. doi: 10.1186/s12906-021-03483-z. BMC Complement Med Ther. 2022. PMID: 35177060 Free PMC article.
-
Lotus essential oil improves neurite elongation and facilitates functional recovery after peripheral nerve injury.Biomed Rep. 2022 Apr;16(4):30. doi: 10.3892/br.2022.1513. Epub 2022 Feb 18. Biomed Rep. 2022. PMID: 35251617 Free PMC article.
-
Isoquercitrin promotes peripheral nerve regeneration through inhibiting oxidative stress following sciatic crush injury in mice.Ann Transl Med. 2019 Nov;7(22):680. doi: 10.21037/atm.2019.11.18. Ann Transl Med. 2019. PMID: 31930081 Free PMC article.
-
Liver X Receptor exerts a protective effect against the oxidative stress in the peripheral nerve.Sci Rep. 2018 Feb 6;8(1):2524. doi: 10.1038/s41598-018-20980-3. Sci Rep. 2018. PMID: 29410501 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

