GDE2 promotes neurogenesis by glycosylphosphatidylinositol-anchor cleavage of RECK
- PMID: 23329048
- PMCID: PMC3644959
- DOI: 10.1126/science.1231921
GDE2 promotes neurogenesis by glycosylphosphatidylinositol-anchor cleavage of RECK
Abstract
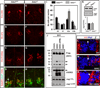
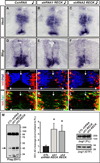
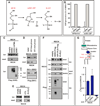
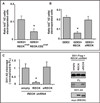
The six-transmembrane protein glycerophosphodiester phosphodiesterase 2 (GDE2) induces spinal motor neuron differentiation by inhibiting Notch signaling in adjacent motor neuron progenitors. GDE2 function requires activity of its extracellular domain that shares homology with glycerophosphodiester phosphodiesterases (GDPDs). GDPDs metabolize glycerophosphodiesters into glycerol-3-phosphate and corresponding alcohols, but whether GDE2 inhibits Notch signaling by this mechanism is unclear. Here, we show that GDE2, unlike classical GDPDs, cleaves glycosylphosphatidylinositol (GPI) anchors. GDE2 GDPD activity inactivates the Notch activator RECK (reversion-inducing cysteine-rich protein with kazal motifs) by releasing it from the membrane through GPI-anchor cleavage. RECK release disinhibits ADAM (a disintegrin and metalloproteinase) protease-dependent shedding of the Notch ligand Delta-like 1 (Dll1), leading to Notch inactivation. This study identifies a previously unrecognized mechanism to initiate neurogenesis that involves GDE2-mediated surface cleavage of GPI-anchored targets to inhibit Dll1-Notch signaling.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

