Functions of the osteocyte network in the regulation of bone mass
- PMID: 23329124
- PMCID: PMC3637644
- DOI: 10.1007/s00441-012-1546-x
Functions of the osteocyte network in the regulation of bone mass
Abstract
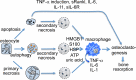
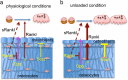
Osteocytes establish an extensive intracellular and extracellular communication system via gap-junction-coupled cell processes and canaliculi throughout bone and the communication system is extended to osteoblasts on the bone surface. The osteocyte network is an ideal mechanosensory system and suitable for mechanotransduction. However, the overall function of the osteocyte network remains to be clarified, since bone resorption is enhanced by osteocyte apoptosis, which is followed by a process of secondary necrosis attributable to the lack of scavengers. The enhanced bone resorption is caused by the release of intracellular content, including immunostimulatory molecules that activate osteoclastogenesis through the canaliculi. Therefore, a mouse model is required in which the osteocyte network is disrupted but in which no bone resorption is induced, in order to evaluate the overall functions of the osteocyte network. One such model is the BCL2 transgenic mouse, in which the osteocyte network, including both intracellular and extracellular networks, is disrupted. Another model is the osteocyte-specific Gja1 knockout mouse, in which intercellular communication through gap junctions is impaired but the canalicular system is intact. Combining the findings from these mouse models with previous histological observations showing the inverse linkage between osteocyte density and bone formation, we conclude that the osteocyte network enhances bone resorption and inhibits bone formation under physiological conditions. Further, studies with BCL2 transgenic mice show that these osteocyte functions are augmented in the unloaded condition. In this condition, Rankl upregulation in osteoblasts and Sost upregulation in osteocytes are, at least in part, responsible for enhanced bone resorption and suppressed bone formation, respectively.
Figures
References
-
- Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, DeSimone D, Bonewald LF, Lafer EM, Sprague E, Schwartz MA, Jiang JX. Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci USA. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. - DOI - PMC - PubMed
-
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. - DOI - PubMed
-
- Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, Rhee Y, Bellido T, Plotkin LI. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27:374–389. doi: 10.1002/jbmr.548. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

