Berberine protects 6-hydroxydopamine-induced human dopaminergic neuronal cell death through the induction of heme oxygenase-1
- PMID: 23329300
- PMCID: PMC3887902
- DOI: 10.1007/s10059-013-2298-5
Berberine protects 6-hydroxydopamine-induced human dopaminergic neuronal cell death through the induction of heme oxygenase-1
Abstract
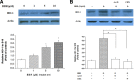
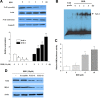
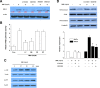
Berberine (BBR) is one of the major alkaloids and has been reported to have a variety of pharmacologic effects, including inhibition of cell cycle progression. Here, we investigated the mechanisms of BBR protection of neuronal cells from cell death induced by the Parkinson's disease-related neurotoxin 6-hydroxydopamine (6-OHDA). Pretreatment of SH-SY5Y cells with BBR significantly reduced 6-OHDAinduced generation of reactive oxygen species (ROS), caspase-3 activation, and subsequent cell death. BBR also upregulated heme oxygenase-1 (HO-1) expression, which conferred protection against 6-OHDA-induced dopaminergic neuron injury and besides, effect of BBR on HO-1 was reversed by siRNA-Nrf2. Furthermore, BBR induced PI3K/Akt and p38 activation, which are involved in the induction of Nrf2 expression and neuroprotection. These results suggest that BBR may be useful as a therapeutic agent for the treatment of dopaminergic neuronal diseases.
Figures



References
-
- Alam J., Cook J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm. Des. 2003;9:2499–2511. - PubMed
-
- Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. - PubMed
-
- Chen X.L., Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004;10:879–891. - PubMed
-
- Chen K., Gunter K., Maines M.D. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J. Neurochem. 2000;75:304–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

