EPR-kinetic isotope effect study of the mechanism of radical-mediated dehydrogenation of an alcohol by the radical SAM enzyme DesII
- PMID: 23329328
- PMCID: PMC3568370
- DOI: 10.1073/pnas.1209446110
EPR-kinetic isotope effect study of the mechanism of radical-mediated dehydrogenation of an alcohol by the radical SAM enzyme DesII
Abstract
The radical S-adenosyl-L-methionine enzyme DesII from Streptomyces venezuelae is able to oxidize the C3 hydroxyl group of TDP-D-quinovose to the corresponding ketone via an α-hydroxyalkyl radical intermediate. It is unknown whether electron transfer from the radical intermediate precedes or follows its deprotonation, and answering this question would offer considerable insight into the mechanism by which the small but important class of radical-mediated alcohol dehydrogenases operate. This question can be addressed by measuring steady-state kinetic isotope effects (KIEs); however, their interpretation is obfuscated by the degree to which the steps of interest limit catalysis. To circumvent this problem, we measured the solvent deuterium KIE on the saturating steady-state concentration of the radical intermediate using electron paramagnetic resonance spectroscopy. The resulting value, 0.22 ± 0.03, when combined with the solvent deuterium KIE on the maximum rate of turnover (V) of 1.8 ± 0.2, yielded a KIE of 8 ± 2 on the net rate constant specifically associated with the α-hydroxyalkyl radical intermediate. This result implies that electron transfer from the radical intermediate does not precede deprotonation. Further analysis of these isotope effects, along with the pH dependence of the steady-state kinetic parameters, likewise suggests that DesII must be in the correct protonation state for initial generation of the α-hydroxyalkyl radical. In addition to providing unique mechanistic insights, this work introduces a unique approach to investigating enzymatic reactions using KIEs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

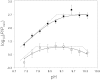
 (○) on pH. The ordinate denotes the base-10 logarithm of the steady-state parameter (P = V or
(○) on pH. The ordinate denotes the base-10 logarithm of the steady-state parameter (P = V or  ) normalized vs. the minimum value
) normalized vs. the minimum value  observed. The results for V are shifted one log unit along the ordinate for presentation purposes. Error bars denote ±1 SE of the observed parameter at the given pH following propagation through the logarithm. Fitted lines and the apparent pKa values were estimated by weighted nonlinear regression as described in
observed. The results for V are shifted one log unit along the ordinate for presentation purposes. Error bars denote ±1 SE of the observed parameter at the given pH following propagation through the logarithm. Fitted lines and the apparent pKa values were estimated by weighted nonlinear regression as described in 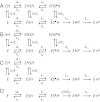
 as the pH is lowered. Intermediates E and
as the pH is lowered. Intermediates E and  denote the binary complexes between DesII and SAM in the unprotonated and protonated states, respectively. TDP-
denote the binary complexes between DesII and SAM in the unprotonated and protonated states, respectively. TDP- (with concentration s). The conversions
(with concentration s). The conversions  and
and  denote collectively the reductive homolysis of SAM and subsequent generation of the substrate radical by the protonated and unprotonated enzyme forms, respectively. The α-hydroxyalkyl radical observed by EPR (23) thus corresponds to both
denote collectively the reductive homolysis of SAM and subsequent generation of the substrate radical by the protonated and unprotonated enzyme forms, respectively. The α-hydroxyalkyl radical observed by EPR (23) thus corresponds to both  and
and  . The net rate constant
. The net rate constant  describes both the electron transfer and deprotonation events of the substrate radical (8→7). Complete release of products and binding of SAM, which is saturating, are described by
describes both the electron transfer and deprotonation events of the substrate radical (8→7). Complete release of products and binding of SAM, which is saturating, are described by  .
.
References
-
- Silverman RB. The Organic Chemistry of Enzyme-Catalyzed Reactions. London, UK: Academic; 2008.
-
- Fitzpatrick PF. Substrate dehydrogenation by flavoproteins. Acc Chem Res. 2001;34(4):299–307. - PubMed
-
- Whittaker JW. Free radical catalysis by galactose oxidase. Chem Rev. 2003;103(6):2347–2363. - PubMed
-
- Whittaker JW. The radical chemistry of galactose oxidase. Arch Biochem Biophys. 2005;433(1):227–239. - PubMed
-
- Frey PA, Magnusson OT. S-Adenosylmethionine: A wolf in sheep’s clothing, or a rich man’s adenosylcobalamin? Chem Rev. 2003;103(6):2129–2148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

