Ocular neovascularization
- PMID: 23329331
- PMCID: PMC3584193
- DOI: 10.1007/s00109-013-0993-5
Ocular neovascularization
Abstract
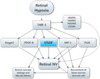
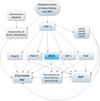
Retinal and choroidal vascular diseases constitute the most common causes of moderate and severe vision loss in developed countries. They can be divided into retinal vascular diseases, in which there is leakage and/or neovascularization (NV) from retinal vessels, and subretinal NV, in which new vessels grow into the normally avascular outer retina and subretinal space. The first category of diseases includes diabetic retinopathy, retinal vein occlusions, and retinopathy of prematurity, and the second category includes neovascular age-related macular degeneration (AMD), ocular histoplasmosis, pathologic myopia, and other related diseases. Retinal hypoxia is a key feature of the first category of diseases resulting in elevated levels of hypoxia-inducible factor-1 (HIF-1) which stimulates expression of vascular endothelial growth factor (VEGF), platelet-derived growth factor-B (PDGF-B), placental growth factor, stromal-derived growth factor-1 and their receptors, as well as other hypoxia-regulated gene products such as angiopoietin-2. Although hypoxia has not been demonstrated as part of the second category of diseases, HIF-1 is elevated and thus the same group of hypoxia-regulated gene products plays a role. Clinical trials have shown that VEGF antagonists provide major benefits for patients with subretinal NV due to AMD and even greater benefits are seen by combining antagonists of VEGF and PDGF-B. It is likely that addition of antagonists of other agents listed above will be tested in the future. Other appealing strategies are to directly target HIF-1 or to use gene transfer to express endogenous or engineered anti-angiogenic proteins. While substantial progress has been made, the future looks even brighter for patients with retinal and choroidal vascular diseases.
Figures



References
-
- Michaelson I. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK. 1948;68:137–180.
-
- Baird A, Esch F, Gospodarowicz D, Guillemin R. Retina- and eye-derived endothelial cell growth factors: partial molecular chariacterization and identity with acidic and basic fibroblast growth factors. Biochemistry. 1985;24:7855–7860. - PubMed
-
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. - PubMed
-
- Plate KH, Breier G, Welch HA, Risau W. Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. - PubMed
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

