Posttranslational modification and quality control
- PMID: 23329792
- PMCID: PMC3566557
- DOI: 10.1161/CIRCRESAHA.112.268706
Posttranslational modification and quality control
Abstract
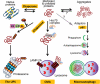
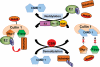
Protein quality control functions to minimize the level and toxicity of misfolded proteins in the cell. Protein quality control is performed by intricate collaboration among chaperones and target protein degradation. The latter is performed primarily by the ubiquitin-proteasome system and perhaps autophagy. Terminally misfolded proteins that are not timely removed tend to form aggregates. Their clearance requires macroautophagy. Macroautophagy serves in intracellular quality control also by selectively segregating defective organelles (eg, mitochondria) and targeting them for degradation by the lysosome. Inadequate protein quality control is observed in a large subset of failing human hearts with a variety of causes, and its pathogenic role has been experimentally demonstrated. Multiple posttranslational modifications can occur to substrate proteins and protein quality control machineries, promoting or hindering the removal of the misfolded proteins. This article highlights recent advances in posttranslational modification-mediated regulation of intracellular quality control mechanisms and its known involvement in cardiac pathology.
Figures
References
-
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

