The practical management of treatment failure in chronic hepatitis C: a summary of current research and management options for refractory patients
- PMID: 23329897
- PMCID: PMC3099312
The practical management of treatment failure in chronic hepatitis C: a summary of current research and management options for refractory patients
Figures
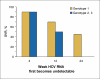
sustained virologic response
hepatitis C virus.
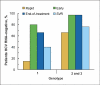
hepatitis C virus.

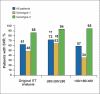
Beck Depression Inventory
Center for Epidemiological Studies Depression Rating Scale
serotonin reuptake inhibitor
Zung Self-Rating Depression Scale Index score.

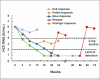
consensus interferon.
Similar articles
-
Enhanced External Counterpulsation (EECP): An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2006;6(5):1-70. Epub 2006 Mar 1. Ont Health Technol Assess Ser. 2006. PMID: 23074496 Free PMC article.
-
A Practical Approach to Refractory Chronic Rhinosinusitis.Otolaryngol Clin North Am. 2017 Feb;50(1):183-198. doi: 10.1016/j.otc.2016.08.014. Otolaryngol Clin North Am. 2017. PMID: 27888913 Review.
-
Resistance to hepatitis C virus. Implications and therapeutic options.Gastroenterol Hepatol. 2017 Aug-Sep;40(7):484-494. doi: 10.1016/j.gastrohep.2017.04.007. Epub 2017 Jun 22. Gastroenterol Hepatol. 2017. PMID: 28647053 English, Spanish.
-
European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults.HIV Med. 2008 Feb;9(2):82-8. doi: 10.1111/j.1468-1293.2007.00535.x. HIV Med. 2008. PMID: 18257771
-
New hepatitis C virus therapies: drug classes and metabolism, drug interactions relevant in the transplant settings, drug options in decompensated cirrhosis, and drug options in end-stage renal disease.Curr Opin Organ Transplant. 2015 Jun;20(3):235-41. doi: 10.1097/MOT.0000000000000198. Curr Opin Organ Transplant. 2015. PMID: 25944238 Review.
Cited by
-
Budget impact and cost-effectiveness analyses of direct-acting antivirals for chronic hepatitis C virus infection in Hong Kong.Eur J Clin Microbiol Infect Dis. 2017 Oct;36(10):1801-1809. doi: 10.1007/s10096-017-2995-7. Epub 2017 May 17. Eur J Clin Microbiol Infect Dis. 2017. PMID: 28516201
-
Comparison of liver biopsies before and after direct-acting antiviral therapy for hepatitis C and correlation with clinical outcome.Sci Rep. 2021 Jul 15;11(1):14506. doi: 10.1038/s41598-021-93881-7. Sci Rep. 2021. PMID: 34267267 Free PMC article.
References
-
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-a2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. - PubMed
-
- Shiffman ML, Suter F, Bacon BR, et al. A randomized controlled trial evaluating 16 and 24 weeks of peginterferon alfa-2a plus ribavirin in patients with HCV genotypes 2 or 3. N Engl J Med. In press.
-
- Shiffman ML, Pappas S, Nyberg S, et al. Peginterferon alfa-2A (PEGASYS) plus ribavirin (COPEGUS) for 16 or 24 weeks in patients with HCV genotype 2 or 3. Final results of the ACCELERATE trial. Presented at the European Association for the Study of Liver 41st Annual Meeting; April 26-30, 2006; Vienna, Austria. Abstract 734.
LinkOut - more resources
Full Text Sources
