Non-ribosomal propeptide precursor in nocardicin A biosynthesis predicted from adenylation domain specificity dependent on the MbtH family protein NocI
- PMID: 23330869
- PMCID: PMC3571714
- DOI: 10.1021/ja307710d
Non-ribosomal propeptide precursor in nocardicin A biosynthesis predicted from adenylation domain specificity dependent on the MbtH family protein NocI
Abstract
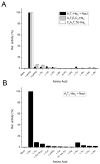
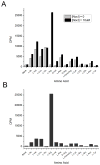
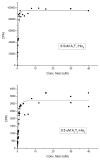
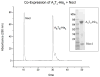
Nocardicin A is a monocyclic β-lactam isolated from the actinomycete Nocardia uniformis that shows moderate antibiotic activity against a broad spectrum of gram-negative bacteria. The monobactams are of renewed interest due to emerging gram-negative strains resistant to clinically available penicillins and cephalosporins. Like isopenicillin N, nocardicin A has a tripeptide core of non-ribosomal origin. Paradoxically, the nocardicin A gene cluster encodes two non-ribosomal peptide synthetases (NRPSs), NocA and NocB, predicted to encode five modules pointing to a pentapeptide precursor in nocardicin A biosynthesis, unless module skipping or other nonlinear reactions are occurring. Previous radiochemical incorporation experiments and bioinformatic analyses predict the incorporation of p-hydroxy-L-phenylglycine (L-pHPG) into positions 1, 3, and 5 and L-serine into position 4. No prediction could be made for position 2. Multidomain constructs of each module were heterologous expressed in Escherichia coli for determination of the adenylation domain (A-domain) substrate specificity using the ATP/PPi exchange assay. Three of the five A-domains, from modules 1, 2, and 4, required the addition of stoichiometric amounts of MbtH family protein NocI to detect exchange activity. On the basis of these analyses, the predicted product of the NocA and NocB NRPSs is L-pHPG-L-Arg-D-pHPG-L-Ser-L-pHPG, a pentapeptide. Despite being flanked by non-proteinogenic amino acids, proteolysis of this pentapeptide by trypsin yields two fragments from cleavage at the C terminus of the L-Arg residue. Thus, a proteolytic step is likely involved in the biosynthesis of nocardicin A, a rare but precedented editing event in the formation of non-ribosomal natural products that is supported by the identification of trypsin-encoding genes in N. uniformis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

