Carbohydrate structure characterization by tandem ion mobility mass spectrometry (IMMS)2
- PMID: 23330948
- PMCID: PMC3633474
- DOI: 10.1021/ac303273z
Carbohydrate structure characterization by tandem ion mobility mass spectrometry (IMMS)2
Abstract
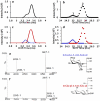
A high resolution ion mobility spectrometer was interfaced to a Synapt G2 high definition mass spectrometer (HDMS) to produce IMMS-IMMS analysis. The hybrid instrument contained an electrospray ionization source, two ion gates, an ambient pressure linear ion mobility drift tube, a quadrupole mass filter, a traveling wave ion mobility spectrometer (TWIMS), and a time-of-flight mass spectrometer. The dual gate drift tube ion mobility spectrometer (DTIMS) could be used to acquire traditional IMS spectra but also could selectively transfer specific mobility selected precursor ions to the Synapt G2 HDMS for mass filtration (quadrupole). The mobility and mass selected ions could then be introduced into a collision cell for fragmentation followed by mobility separation of the fragment ions with the traveling wave ion mobility spectrometer. These mobility separated fragment ions are finally mass analyzed using a time-of-flight mass spectrometer. This results in an IMMS-IMMS analysis and provides a method to evaluate the isomeric heterogeneity of precursor ions by both DTIMS and TWIMS to acquire a mobility-selected and mass-filtered fragmentation pattern and to additionally obtain traveling wave ion mobility spectra of the corresponding product ions. This new IMMS(2) instrument enables the structural diversity of carbohydrates to be studied in greater detail. The physical separation of isomeric oligosaccharide mixtures was achieved by both DTIMS and TWIMS, with DTIMS demonstrating higher resolving power (70-80) than TWIMS (30-40). Mobility selected MS/MS spectra were obtained, and TWIMS evaluation of product ions showed that isomeric forms of fragment ions existed for identical m/z values.
Figures






Similar articles
-
Determining the isomeric heterogeneity of neutral oligosaccharide-alditols of bovine submaxillary mucin using negative ion traveling wave ion mobility mass spectrometry.Anal Chem. 2015 Feb 17;87(4):2228-35. doi: 10.1021/ac503754k. Epub 2015 Jan 29. Anal Chem. 2015. PMID: 25594283
-
Evaluation of ion mobility-mass spectrometry for determining the isomeric heterogeneity of oligosaccharide-alditols derived from bovine submaxillary mucin.Int J Mass Spectrom. 2013 Oct 15;352:9-18. doi: 10.1016/j.ijms.2013.07.015. Int J Mass Spectrom. 2013. PMID: 24634605 Free PMC article.
-
Ion mobility-mass spectrometry.J Mass Spectrom. 2008 Jan;43(1):1-22. doi: 10.1002/jms.1383. J Mass Spectrom. 2008. PMID: 18200615 Review.
-
Separation of isomeric cereal-derived arabinoxylan-oligosaccharides by collision induced dissociation-travelling wave ion mobility spectrometry-tandem mass spectrometry (CID-TWIMS-MS/MS).Food Chem. 2022 Jan 1;366:130544. doi: 10.1016/j.foodchem.2021.130544. Epub 2021 Jul 5. Food Chem. 2022. PMID: 34314932
-
[Applications of ion mobility-mass spectrometry in the chemical analysis in traditional Chinese medicines].Se Pu. 2022 Sep;40(9):782-787. doi: 10.3724/SP.J.1123.2022.01028. Se Pu. 2022. PMID: 36156624 Free PMC article. Review. Chinese.
Cited by
-
GlycoMob: an ion mobility-mass spectrometry collision cross section database for glycomics.Glycoconj J. 2016 Jun;33(3):399-404. doi: 10.1007/s10719-015-9613-7. Epub 2015 Aug 28. Glycoconj J. 2016. PMID: 26314736
-
Linkage and anomeric differentiation in trisaccharides by sequential fragmentation and variable-wavelength infrared photodissociation.J Am Soc Mass Spectrom. 2015 Feb;26(2):359-68. doi: 10.1007/s13361-014-1025-6. Epub 2014 Dec 10. J Am Soc Mass Spectrom. 2015. PMID: 25492690
-
Travelling-wave ion mobility and negative ion fragmentation of high-mannose N-glycans.J Mass Spectrom. 2016 Mar;51(3):219-35. doi: 10.1002/jms.3738. J Mass Spectrom. 2016. PMID: 26956389 Free PMC article.
-
Are the Gas-Phase Structures of Molecular Elephants Enduring or Ephemeral? Results from Time-Dependent, Tandem Ion Mobility.Anal Chem. 2023 Jun 27;95(25):9589-9597. doi: 10.1021/acs.analchem.3c01222. Epub 2023 Jun 9. Anal Chem. 2023. PMID: 37294019 Free PMC article.
-
Differentiation of Isomeric, Nonseparable Carbohydrates Using Tandem-Trapped Ion Mobility Spectrometry-Mass Spectrometry.Anal Chem. 2023 Jan 17;95(2):747-757. doi: 10.1021/acs.analchem.2c02844. Epub 2022 Dec 22. Anal Chem. 2023. PMID: 36547374 Free PMC article.
References
-
- Cohen MJ, Karasek FW. J. Chromatogr. Sci. 1970;8:330–337.
-
- Griffin GW, Dzidic I, Carroll DI, Stillwell RN, Horning EC. Anal. Chem. 1973;45:1204–1209.
-
- Carroll DI, Dzidic I, Stillwell RN, Horning EC. Anal. Chem. 1975;47:1956–1959. - PubMed
-
- Karasek FW, Kim SH, Hill HH., Jr Anal. Chem. 1976;48:1133–1137.
-
- Spangler GE, Lawless PA. Anal. Chem. 1978;50:884–892.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

