The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates
- PMID: 23331006
- PMCID: PMC4419808
- DOI: 10.2174/1566524011313030003
The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates
Abstract
CA 19-9 and CEA are the most commonly used biomarkers for diagnosis and management of patients with pancreatic cancer. Since the original compendium by Steinberg in 1990, numerous studies have reported the use of CA 19-9 and, to a lesser extent, CEA in the diagnosis of pancreatic cancer. Here we update an evaluation of the accuracy of CA 19-9 and CEA, and, unlike previous reviews, focus on discrimination between malignant and benign disease instead of normal controls. In 57 studies involving 3,285 pancreatic carcinoma cases, the combined sensitivity of CA 19-9 was 78.2% and in 37 studies involving 1,882 cases with benign pancreatic disease the specificity of CA 19-9 was 82.8%. From the combined analysis of studies reporting CEA, the sensitivity was 44.2% (1,324 cases) and the specificity was 84.8% (656 cases). These measurements more appropriately reflect the expected biomarker accuracy in the differential diagnosis of patients with periampullary diseases. We also present a summary of the use of CA 19-9 as a prognostic tool and evaluate CA 19-9 diagnostic and prognostic utility in a 10-year, single institution experience.
Figures

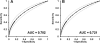
References
-
- Koprowski H, et al. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212(4490):53–5. - PubMed
-
- Koprowski H, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5(6):957–71. - PubMed
-
- Del Villano BC, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983;29(3):549–52. - PubMed
-
- Homma T, Tsuchiya R. The study of the mass screening of persons without symptoms and of the screening of outpatients with gastrointestinal complaints or icterus for pancreatic cancer in Japan, using CA19-9 and elastase-1 or ultrasonography. Int J Pancreatol. 1991;9:119–24. - PubMed
-
- Kim JE, et al. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19(2):182–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
