Elastomeric negative acoustic contrast particles for affinity capture assays
- PMID: 23331264
- PMCID: PMC3621144
- DOI: 10.1021/ac3029344
Elastomeric negative acoustic contrast particles for affinity capture assays
Abstract
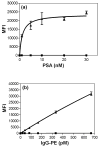
This report describes the development of elastomeric capture microparticles (ECμPs) and their use with acoustophoretic separation to perform microparticle assays via flow cytometry.We have developed simple methods to form ECμPs by cross-linking droplets of common commercially available silicone precursors in suspension followed by surface functionalization with biomolecular recognition reagents. The ECμPs are compressible particles that exhibit negative acoustic contrast in ultrasound when suspended in aqueous media, blood serum, or diluted blood. In this study, these particles have been functionalized with antibodies to bind prostate specific antigen and immunoglobulin (IgG). Specific separation of the ECμPs from blood cells is achieved by flowing them through a microfluidic acoustophoretic device that uses an ultrasonic standing wave to align the blood cells, which exhibit positive acoustic contrast, at a node in the acoustic pressure distribution while aligning the negative acoustic contrast ECμPs at the antinodes. Laminar flow of the separated particles to downstream collection ports allows for collection of the separated negative contrast (ECμPs) and positive contrast particles (cells). Separated ECμPs were analyzed via flow cytometry to demonstrate nanomolar detection for prostate specific antigen in aqueous buffer and picomolar detection for IgG in plasma and diluted blood samples. This approach has potential applications in the development of rapid assays that detect the presence of low concentrations of biomarkers in a number of biological sample types.
Figures




References
-
- Laurell T, Petersson F, Nilsson A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chemical Society Reviews. 2007;36(3):492–506. - PubMed
-
- Nam J, Lim H, Kim D, Shin S. Separation of platelets from whole blood using standing surface acoustic waves in a microchannel. Lab on a Chip. 2011;11(19):3361–3364. - PubMed
-
- Meng Z, Veenstra TD. Targeted mass spectrometry approaches for protein biomarker verification. Journal of Proteomics. 2011;74(12):2650–2659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

