Pheromonal bile acid 3-ketopetromyzonol sulfate primes the neuroendocrine system in sea lamprey
- PMID: 23331321
- PMCID: PMC3599739
- DOI: 10.1186/1471-2202-14-11
Pheromonal bile acid 3-ketopetromyzonol sulfate primes the neuroendocrine system in sea lamprey
Abstract
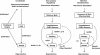
Background: Vertebrate pheromones are known to prime the endocrine system, especially the hypothalamic-pituitary-gonadal (HPG) axis. However, no known pheromone molecule has been shown to modulate directly the synthesis or release of gonadotropin releasing hormone (GnRH), the main regulator of the HPG axis. We selected sea lamprey (Petromyzon marinus) as a model system to determine whether a single pheromone component alters the output of GnRH.Sea lamprey male sex pheromones contain a main component, 7α, 12α, 24-trihydroxy-5α-cholan-3-one 24-sulfate (3 keto-petromyzonol sulfate or 3kPZS), which has been shown to modulate behaviors of mature females. Through a series of experiments, we tested the hypothesis that 3kPZS modulates both synthesis and release of GnRH, and subsequently, HPG output in immature sea lamprey.
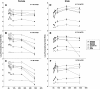
Results: The results showed that natural male pheromone mixtures induced differential steroid responses but facilitated sexual maturation in both sexes of immature animals (χ(2) = 5.042, dF = 1, p < 0.05). Exposure to 3kPZS increased plasma 15α-hydroxyprogesterone (15α-P) concentrations (one-way ANOVA, p < 0.05) and brain gene expressions (genes examined: three lamprey (l) GnRH-I transcripts, lGnRH-III, Jun and Jun N-terminal kinase (JNK); one-way ANOVA, p < 0.05), but did not alter the number of GnRH neurons in the hypothalamus in immature animals. In addition, 3kPZS treatments increased lGnRH peptide concentrations in the forebrain and modulated their levels in plasma. Overall, 3kPZS modulation of HPG axis is more pronounced in immature males than in females.
Conclusions: We conclude that a single male pheromone component primes the HPG axis in immature sea lamprey in a sexually dimorphic manner.
Figures
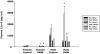
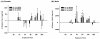

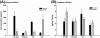
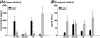
Similar articles
-
An anti-steroidogenic inhibitory primer pheromone in male sea lamprey (Petromyzon marinus).Gen Comp Endocrinol. 2013 Aug 1;189:24-31. doi: 10.1016/j.ygcen.2013.04.023. Epub 2013 Apr 30. Gen Comp Endocrinol. 2013. PMID: 23644156
-
Waterborne pheromones modulate gonadotropin-inhibitory hormone levels in sea lamprey (Petromyzon marinus).Gen Comp Endocrinol. 2020 Mar 1;288:113358. doi: 10.1016/j.ygcen.2019.113358. Epub 2019 Dec 14. Gen Comp Endocrinol. 2020. PMID: 31837303
-
Evidence that progestins play an important role in spermiation and pheromone production in male sea lamprey (Petromyzon marinus).Gen Comp Endocrinol. 2015 Feb 1;212:17-27. doi: 10.1016/j.ygcen.2015.01.008. Epub 2015 Jan 23. Gen Comp Endocrinol. 2015. PMID: 25623147
-
Petromyzonol sulfate and its derivatives: the chemoattractants of the sea lamprey.Bioessays. 2005 Feb;27(2):222-8. doi: 10.1002/bies.20155. Bioessays. 2005. PMID: 15666352 Review.
-
lGnRH-III -- a promising candidate for anticancer drug development.Protein Pept Lett. 2013 Apr;20(4):439-49. Protein Pept Lett. 2013. PMID: 23016589 Review.
Cited by
-
Chemical cues and pheromones in the sea lamprey (Petromyzon marinus).Front Zool. 2015 Nov 25;12:32. doi: 10.1186/s12983-015-0126-9. eCollection 2015. Front Zool. 2015. PMID: 26609313 Free PMC article. Review.
-
Endocrine Regulation of Maturation and Sex Change in Groupers.Cells. 2022 Feb 27;11(5):825. doi: 10.3390/cells11050825. Cells. 2022. PMID: 35269447 Free PMC article. Review.
-
Investigations of novel unsaturated bile salts of male sea lamprey as potential chemical cues.J Chem Ecol. 2014 Oct;40(10):1152-60. doi: 10.1007/s10886-014-0511-4. Epub 2014 Oct 30. J Chem Ecol. 2014. PMID: 25355633
-
Theory and Application of Semiochemicals in Nuisance Fish Control.J Chem Ecol. 2016 Jul;42(7):698-715. doi: 10.1007/s10886-016-0729-4. Epub 2016 Jul 14. J Chem Ecol. 2016. PMID: 27417504 Review.
-
Integrative neuro-endocrine pathways in the control of reproduction in lamprey: a brief review.Front Endocrinol (Lausanne). 2013 Oct 18;4:151. doi: 10.3389/fendo.2013.00151. Front Endocrinol (Lausanne). 2013. PMID: 24151489 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

