Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: Individual patient data from Japanese randomized, placebo-controlled trials
- PMID: 23331490
- PMCID: PMC7657198
- DOI: 10.1111/cas.12105
Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: Individual patient data from Japanese randomized, placebo-controlled trials
Abstract
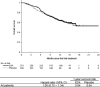
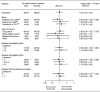
Erythropoiesis-stimulating agents (ESA) reduce the need for transfusions and improve the quality of life in patients receiving chemotherapy, but several clinical trials have suggested that ESA might have a negative impact on survival. To evaluate the efficacy and safety of ESA, epoetin beta and darbepoetin alfa, including their impact on overall survival and thromboembolic events, we conducted an individual data-based meta-analysis of three randomized, placebo-controlled trials studying Japanese patients with chemotherapy-induced anemia. All trials were conducted in compliance with Good Clinical Practice. A total of 511 patients with solid tumor or lymphoma (epoetin beta or darbepoetin alfa, n = 273; placebo, n = 238) were included. The ESA significantly reduced the risk of transfusion (relative risk, 0.47; 95% confidence interval, 0.29-0.76). No significant effect of the ESA on overall survival was observed (unadjusted hazard ratio, 1.00; 95% confidence interval, 0.75-1.34). A prespecified subgroup analysis showed no strong interaction between the baseline hemoglobin concentration and the effect of ESA on overall survival. Among the ESA-treated patients, the highest hemoglobin achieved during the treatment period in each patient had no impact on mortality. No increase in thromboembolic events was observed in the ESA-treated patients (0.7% vs 1.7% placebo). The ESA reduced the risk of transfusion without a negative impact on the survival of patients with chemotherapy-induced anemia.
© 2013 Japanese Cancer Association.
Figures
Similar articles
-
Use of erythropoiesis-stimulating agents among chemotherapy patients with hemoglobin exceeding 12 grams per deciliter.J Manag Care Pharm. 2008 Nov-Dec;14(9):858-69. doi: 10.18553/jmcp.2008.14.9.858. J Manag Care Pharm. 2008. PMID: 19006442 Free PMC article.
-
Use and prescribing patterns for erythropoiesis-stimulating agents in inpatient and outpatient hospital settings.Am J Health Syst Pharm. 2008 Sep 15;65(18):1711-9. doi: 10.2146/ajhp070526. Am J Health Syst Pharm. 2008. PMID: 18768997
-
Darbepoetin alfa for treating chemotherapy-induced anemia in patients with a baseline hemoglobin level < 10 g/dL versus > or = 10 g/dL: an exploratory analysis from a randomized, double-blind, active-controlled trial.BMC Cancer. 2009 Sep 3;9:311. doi: 10.1186/1471-2407-9-311. BMC Cancer. 2009. PMID: 19728887 Free PMC article. Clinical Trial.
-
Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update.Blood. 2008 Jan 1;111(1):25-41. doi: 10.1182/blood-2007-08-109488. Epub 2007 Oct 22. Blood. 2008. PMID: 17954703 Review.
-
Use of erythropoietin-stimulating agents in breast cancer patients: a risk review.Am J Health Syst Pharm. 2009 Jul 1;66(13):1180-5. doi: 10.2146/ajhp080214. Am J Health Syst Pharm. 2009. PMID: 19535656 Review.
Cited by
-
Onco-nephrology: an appraisal of the cancer and chronic kidney disease links.Nephrol Dial Transplant. 2015 Dec;30(12):1979-88. doi: 10.1093/ndt/gfu387. Epub 2015 Feb 3. Nephrol Dial Transplant. 2015. PMID: 25648910 Free PMC article. Review.
-
Anemia and Iron Deficiency in Cancer Patients: Role of Iron Replacement Therapy.Pharmaceuticals (Basel). 2018 Sep 30;11(4):94. doi: 10.3390/ph11040094. Pharmaceuticals (Basel). 2018. PMID: 30274354 Free PMC article. Review.
-
Erythropoietin enhances Kupffer cell number and activity in the challenged liver.Sci Rep. 2017 Sep 4;7(1):10379. doi: 10.1038/s41598-017-11082-7. Sci Rep. 2017. PMID: 28871174 Free PMC article.
-
Erythropoietin Receptor (EPOR) Signaling in the Osteoclast Lineage Contributes to EPO-Induced Bone Loss in Mice.Int J Mol Sci. 2022 Oct 10;23(19):12051. doi: 10.3390/ijms231912051. Int J Mol Sci. 2022. PMID: 36233351 Free PMC article.
-
Erythropoiesis-stimulating agents in the management of cancer patients with anemia: a meta-analysis.Chin J Cancer Res. 2014 Jun;26(3):268-76. doi: 10.3978/j.issn.1000-9604.2014.05.03. Chin J Cancer Res. 2014. PMID: 25035653 Free PMC article.
References
-
- Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, Epoetin Alfa Study Group . Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double‐blind, placebo‐controlled trial. J Clin Oncol 2001; 19: 2865–74. - PubMed
-
- Osterborg A, Brandberg Y, Molostova V et al Randomized, double‐blind, placebo‐controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 2002; 20: 2486–94. - PubMed
-
- Vansteenkiste J, Pirker R, Massuti B et al Double‐blind, placebo‐controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002; 94: 1211–20. - PubMed
-
- Leyland‐Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol 2003; 4: 459–60. - PubMed
-
- Henke M, Laszig R, Rübe C et al Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double‐blind, placebo‐controlled trial. Lancet 2003; 362: 1255–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

