Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: Individual patient data from Japanese randomized, placebo-controlled trials
- PMID: 23331490
- PMCID: PMC7657198
- DOI: 10.1111/cas.12105
Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: Individual patient data from Japanese randomized, placebo-controlled trials
Abstract
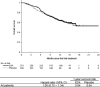
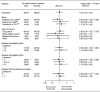
Erythropoiesis-stimulating agents (ESA) reduce the need for transfusions and improve the quality of life in patients receiving chemotherapy, but several clinical trials have suggested that ESA might have a negative impact on survival. To evaluate the efficacy and safety of ESA, epoetin beta and darbepoetin alfa, including their impact on overall survival and thromboembolic events, we conducted an individual data-based meta-analysis of three randomized, placebo-controlled trials studying Japanese patients with chemotherapy-induced anemia. All trials were conducted in compliance with Good Clinical Practice. A total of 511 patients with solid tumor or lymphoma (epoetin beta or darbepoetin alfa, n = 273; placebo, n = 238) were included. The ESA significantly reduced the risk of transfusion (relative risk, 0.47; 95% confidence interval, 0.29-0.76). No significant effect of the ESA on overall survival was observed (unadjusted hazard ratio, 1.00; 95% confidence interval, 0.75-1.34). A prespecified subgroup analysis showed no strong interaction between the baseline hemoglobin concentration and the effect of ESA on overall survival. Among the ESA-treated patients, the highest hemoglobin achieved during the treatment period in each patient had no impact on mortality. No increase in thromboembolic events was observed in the ESA-treated patients (0.7% vs 1.7% placebo). The ESA reduced the risk of transfusion without a negative impact on the survival of patients with chemotherapy-induced anemia.
© 2013 Japanese Cancer Association.
Figures
References
-
- Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, Epoetin Alfa Study Group . Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double‐blind, placebo‐controlled trial. J Clin Oncol 2001; 19: 2865–74. - PubMed
-
- Osterborg A, Brandberg Y, Molostova V et al Randomized, double‐blind, placebo‐controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 2002; 20: 2486–94. - PubMed
-
- Vansteenkiste J, Pirker R, Massuti B et al Double‐blind, placebo‐controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002; 94: 1211–20. - PubMed
-
- Leyland‐Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol 2003; 4: 459–60. - PubMed
-
- Henke M, Laszig R, Rübe C et al Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double‐blind, placebo‐controlled trial. Lancet 2003; 362: 1255–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

