Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'
- PMID: 23331510
- PMCID: PMC3556334
- DOI: 10.1186/1471-2334-13-22
Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'
Abstract
Background: Vitamin D enhances host protective immune responses to Mycobacterium tuberculosis by suppressing Interferon-gamma (IFN-g) and reducing disease associated inflammation in the host. The objectives of this study were to determine whether vitamin D supplementation to patients with tuberculosis (TB) could influence recovery.
Methods: Two hundred and fifty nine patients with pulmonary TB were randomized to receive either 600,000 IU of Intramuscular vitamin D3 or placebo for 2 doses. Assessments were performed at 4, 8 and 12 weeks. Early secreted and T cell activated 6 kDa (ESAT6) and Mycobacterium tuberculosis sonicate (MTBs) antigen induced whole blood stimulated IFN-g responses were measured at 0 and 12 weeks. Statistical comparisons between outcome variables at 0 and 12 weeks were performed using Student's t-test and Chi2 tests.
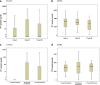
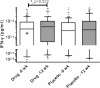
Results: After 12 weeks, the vitamin D supplemented arm demonstrated significantly greater mean weight gain (kg)+3.75, (3.16-4.34) versus+2.61 (95% CI 1.99-3.23) p 0.009 and lesser residual disease by chest radiograph; number of zones involved 1.35 v/s 1.82 p 0.004 (95% CI 0.15, 0.79) and 50% or greater reduction in cavity size 106 (89.8%) v/s 111 (94.8%), p 0.035. Vitamin D supplementation led to significant increase in MTBs-induced IFN-g secretion in patients with baseline 'Deficient' 25-hydroxyvitamin D serum levels (p 0.021).
Conclusions: Supplementation with high doses of vitamin D accelerated clinical, radiographic improvement in all TB patients and increased host immune activation in patients with baseline 'Deficient' serum vitamin D levels. These results suggest a therapeutic role for vitamin D in the treatment of TB.
Trial registration: ClinicalTrials.gov; No. NCT01130311; URL: clinicaltrials.gov.
Figures
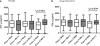
References
-
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. - PubMed
-
- Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

