Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective
- PMID: 23331918
- PMCID: PMC3918746
- DOI: 10.1111/ede.12015
Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective
Abstract
The tetrapod auditory system transmits sound through the outer and middle ear to the organ of Corti or other sound pressure receivers of the inner ear where specialized hair cells translate vibrations of the basilar membrane into electrical potential changes that are conducted by the spiral ganglion neurons to the auditory nuclei. In other systems, notably the vertebrate limb, a detailed connection between the evolutionary variations in adaptive morphology and the underlying alterations in the genetic basis of development has been partially elucidated. In this review, we attempt to correlate evolutionary and partially characterized molecular data into a cohesive perspective of the evolution of the mammalian organ of Corti out of the tetrapod basilar papilla. We propose a stepwise, molecularly partially characterized transformation of the ancestral, vestibular developmental program of the vertebrate ear. This review provides a framework to decipher both discrete steps in development and the evolution of unique functional adaptations of the auditory system. The combined analysis of evolution and development establishes a powerful cross-correlation where conclusions derived from either approach become more meaningful in a larger context which is not possible through exclusively evolution or development centered perspectives. Selection may explain the survival of the fittest auditory system, but only developmental genetics can explain the arrival of the fittest auditory system. [Modified after (Wagner 2011)].
© 2013 Wiley Periodicals, Inc.
Figures
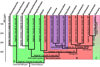
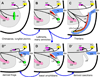
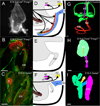



References
-
- Ahn KJ, Passero F, Jr, Crenshaw EB., 3rd Otic mesenchyme expression of Cre recombinase directed by the inner ear enhancer of the Brn4/Pou3f4 gene. Genesis. 2009;47(3):137–141. - PubMed
-
- Amemiya CT, Powers TP, Prohaska SJ, Grimwood J, Schmutz J, Dickson M, Miyake T, Schoenborn MA, Myers RM, Ruddle FH, Stadler PF. Complete HOX cluster characterization of the coelacanth provides further evidence for slow evolution of its genome. Proc Natl Acad Sci U S A. 2010;107(8):3622–3627. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

