Efficient retrograde transport of pseudorabies virus within neurons requires local protein synthesis in axons
- PMID: 23332155
- PMCID: PMC3552305
- DOI: 10.1016/j.chom.2012.10.021
Efficient retrograde transport of pseudorabies virus within neurons requires local protein synthesis in axons
Abstract
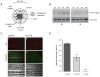
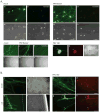
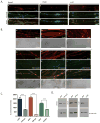
After replicating in epithelial cells, alphaherpesviruses such as pseudorabies virus (PRV) invade axons of peripheral nervous system neurons and undergo retrograde transport toward the distant cell bodies. Although several viral proteins engage molecular motors to facilitate transport, the initial steps and neuronal responses to infection are poorly understood. Using compartmented neuron cultures to physically separate axon infection from cell bodies, we found that PRV infection induces local protein synthesis in axons, including proteins involved in cytoskeletal remodeling, intracellular trafficking, signaling, and metabolism. This rapid translation of axonal mRNAs is required for efficient PRV retrograde transport and infection of cell bodies. Furthermore, induction of axonal damage, which also induces local protein synthesis, prior to infection reduces virion trafficking, suggesting that host damage signals and virus particles compete for retrograde transport. Thus, similar to axonal damage, virus infection induces local protein translation in axons, and viruses likely exploit this response for invasion.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

