Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy
- PMID: 23332170
- PMCID: PMC8970215
- DOI: 10.1016/j.neurobiolaging.2012.12.011
Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy
Abstract
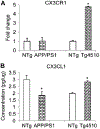
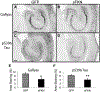
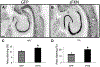
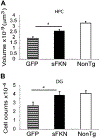
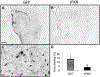
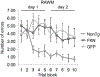
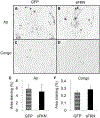
Alzheimer's disease is characterized by amyloid plaques, neurofibrillary tangles, glial activation, and neurodegeneration. In mouse models, inflammatory activation of microglia accelerates tau pathology. The chemokine fractalkine serves as an endogenous neuronal modulator to quell microglial activation. Experiments with fractalkine receptor null mice suggest that fractalkine signaling diminishes tau pathology, but exacerbates amyloid pathology. Consistent with this outcome, we report here that soluble fractalkine overexpression using adeno-associated viral vectors significantly reduced tau pathology in the rTg4510 mouse model of tau deposition. Furthermore, this treatment reduced microglial activation and appeared to prevent neurodegeneration normally found in this model. However, in contrast to studies with fractalkine receptor null mice, parallel studies in an APP/PS1 model found no effect of increased fractalkine signaling on amyloid deposition. These data argue that agonism at fractalkine receptors might be an excellent target for therapeutic intervention in tauopathies, including those associated with amyloid deposition.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement
The authors have no conflicts of interest.
Figures


Similar articles
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Genetically enhancing the expression of chemokine domain of CX3CL1 fails to prevent tau pathology in mouse models of tauopathy.J Neuroinflammation. 2018 Sep 25;15(1):278. doi: 10.1186/s12974-018-1310-6. J Neuroinflammation. 2018. PMID: 30253780 Free PMC article.
-
CCL2 Overexpression in the Brain Promotes Glial Activation and Accelerates Tau Pathology in a Mouse Model of Tauopathy.Front Immunol. 2020 May 20;11:997. doi: 10.3389/fimmu.2020.00997. eCollection 2020. Front Immunol. 2020. PMID: 32508844 Free PMC article.
-
CNS-Wide over Expression of Fractalkine Improves Cognitive Functioning in a Tauopathy Model.J Neuroimmune Pharmacol. 2019 Jun;14(2):312-325. doi: 10.1007/s11481-018-9822-5. Epub 2018 Nov 29. J Neuroimmune Pharmacol. 2019. PMID: 30499006 Free PMC article.
-
The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies.Alzheimers Dement. 2016 Oct;12(10):1040-1050. doi: 10.1016/j.jalz.2016.09.001. Epub 2016 Sep 26. Alzheimers Dement. 2016. PMID: 27686274 Review.
Cited by
-
The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders.Cells. 2020 Oct 13;9(10):2277. doi: 10.3390/cells9102277. Cells. 2020. PMID: 33065974 Free PMC article. Review.
-
Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis.Nat Rev Neurosci. 2019 Feb;20(2):94-108. doi: 10.1038/s41583-018-0113-1. Nat Rev Neurosci. 2019. PMID: 30643230 Free PMC article. Review.
-
Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer's pathology.PLoS One. 2013 Sep 12;8(9):e75713. doi: 10.1371/journal.pone.0075713. eCollection 2013. PLoS One. 2013. PMID: 24069439 Free PMC article.
-
Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice.J Neurosci. 2014 Jul 9;34(28):9222-34. doi: 10.1523/JNEUROSCI.1132-14.2014. J Neurosci. 2014. PMID: 25009256 Free PMC article.
-
Recent Advances in the Study of Bipolar/Rod-Shaped Microglia and their Roles in Neurodegeneration.Front Aging Neurosci. 2017 May 4;9:128. doi: 10.3389/fnagi.2017.00128. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28522972 Free PMC article.
References
-
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, Weiner MF, Farlow MR, Sano M, Grundman M, Thal LJ, 2000. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology 54, 588–593. - PubMed
-
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ, 2003. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289, 2819–2826. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T, 2000. Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421. - PMC - PubMed
-
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D, 2006. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat. Protoc 1,1671–1679. - PubMed
-
- Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM, 2001. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 891, 42–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

