Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers
- PMID: 23332364
- PMCID: PMC3622225
- DOI: 10.1016/S1474-4422(12)70291-0
Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers
Abstract
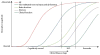
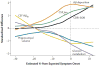
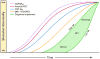
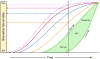
In 2010, we put forward a hypothetical model of the major biomarkers of Alzheimer's disease (AD). The model was received with interest because we described the temporal evolution of AD biomarkers in relation to each other and to the onset and progression of clinical symptoms. Since then, evidence has accumulated that supports the major assumptions of this model. Evidence has also appeared that challenges some of our assumptions, which has allowed us to modify our original model. Refinements to our model include indexing of individuals by time rather than clinical symptom severity; incorporation of interindividual variability in cognitive impairment associated with progression of AD pathophysiology; modifications of the specific temporal ordering of some biomarkers; and recognition that the two major proteinopathies underlying AD biomarker changes, amyloid β (Aβ) and tau, might be initiated independently in sporadic AD, in which we hypothesise that an incident Aβ pathophysiology can accelerate antecedent limbic and brainstem tauopathy.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Dr. Jack serves on scientific advisory boards for Elan/Janssen AI, Bristol Meyer Squibb, Eli Lilly & Company, GE Healthcare, Siemens, and Eisai Inc.; receives research support from Baxter International Inc., Allon Therapeutics, Inc., the NIH/NIA, and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation; and holds stock in Johnson & Johnson. Dr. Knopman serves as Deputy Editor for Neurology®; served on a data safety monitoring board for Eli Lilly and Company; served as a consultant for Elan/Janssen. Dr. Jagust served as a consultant to GE Healthcare, which manufactures flutemetamol, and collaborates with Avid Radiopharmaceuticals, which manufactures florbetapir, through the Alzheimer’s Disease Neuroimaging Initiative. Dr. Weiner serves on the advisory boards for Elan/Wyeth, Novartis, Banner, Lilly, VACO, Biogen Idec, Araclon and Pfizer; serves as a consultant to Elan/Wyeth, Novartis, Forest, Ispen, Daiichi Sankyo, Inc., Astra Zeneca, Araclon, Pfizer, TauRx Therapeutics LTD, Bayer, Biogen Idec, Exonhit Therapeutics, Servier, Synarc; received honoraria from American Academy of Neurology, ipsen, NeuroVigil, Inc., and Insitut Catala de Neurociencies Aplicades; receives research funding from Merck and Avid; owns stock in Synarc and Elan; and serves on the editorial advisory board for Alzheimer’s and Dementia, and MRI. Dr. Aisen serves on a scientific advisory board for NeuroPhage; serves as a consultant to Elan Corporation, Wyeth, Eisai Inc., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Abbott, Pfizer Inc., Novartis, Bayer, Astellas, Dainippon, Biomarin, Solvay, Otsuka, Daiichi, AstraZeneca, Janssen and Medivation Inc.; receives research support from Pfizer Inc., and Baxter International Inc.; and has received stock options from Medivation Inc., and NeuroPhage. Dr. Petersen serves on scientific advisory boards for the Alzheimer’s Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc (Wyeth), and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA. Dr. Shaw serves on the technical advisory board for Saladax Biomedical. Dr. Vemuri, Ms. Wiste, Mr. Weigand, Mr. Lesnick, and Dr. Pankratz report no disclosures. Dr. Trojanowski serves as an Associate Editor of Alzheimer’s & Dementia; may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-Inventor and he received revenue from the sale of Avid to Eli Lily as co-inventor on imaging related patents submitted by the University of Pennsylvania; receives research support from the NIH, Bristol Myer Squib, AstraZenica and several non-profits. Dr. Donohue has served as consultant to Bristol-Meyers Squibb.
Figures


Comment in
-
Biomarkers for Alzheimer's: the sequel of an original model.Lancet Neurol. 2013 Feb;12(2):126-8. doi: 10.1016/S1474-4422(12)70305-8. Lancet Neurol. 2013. PMID: 23332358 No abstract available.
References
-
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. - PubMed
-
- Li G, Sokal I, Quinn JF, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. - PubMed
-
- Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. - PubMed
-
- Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

