Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials
- PMID: 23332990
- PMCID: PMC4552179
- DOI: 10.1053/j.ajkd.2012.11.047
Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials
Abstract
Background: Patients undergoing maintenance hemodialysis frequently exhibit poor mental health. We studied the effects of frequent in-center and nocturnal hemodialysis on depressive symptoms and self-reported mental health.
Study design: 1-year randomized controlled clinical trials.
Setting & participants: Hemodialysis centers in the United States and Canada. 332 patients were randomly assigned to frequent (6-times-weekly) compared with conventional (3-times-weekly) hemodialysis in the Frequent Hemodialysis Network (FHN) Daily (n = 245) and Nocturnal (n = 87) Trials.
Intervention: The Daily Trial was a trial of frequent (6-times-weekly) compared with conventional (3-times-weekly) in-center hemodialysis. The Nocturnal Trial assigned patients to either frequent nocturnal (6-times-weekly) hemodialysis or conventional (3-times-weekly) hemodialysis.
Outcomes: Self-reported depressive symptoms and mental health.
Measurements: Beck Depression Inventory and the mental health composite score and emotional subscale of the RAND 36-Item Health Survey at baseline and 4 and 12 months. The mental health composite score is derived by summarizing these domains of the RAND 36-Item Health Survey: emotional, role emotional, energy/fatigue, and social functioning scales.
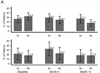
Results: In the Daily Trial, participants randomly assigned to frequent compared with conventional in-center hemodialysis showed no significant change over 12 months in adjusted mean Beck Depression Inventory score (-1.9 ± 0.7 vs -0.6 ± 0.7; P = 0.2), but experienced clinically significant improvements in adjusted mean mental health composite (3.7 ± 0.9 vs 0.2 ± 1.0; P = 0.007) and emotional subscale (5.2 ± 1.6 vs -0.3 ± 1.7; P = 0.01) scores. In the Nocturnal Trial, there were no significant changes in the same metrics in participants randomly assigned to nocturnal compared with conventional hemodialysis.
Limitations: Trial interventions were not blinded.
Conclusions: Frequent in-center hemodialysis, as compared with conventional in-center hemodialysis, improved self-reported general mental health. Changes in self-reported depressive symptoms were not statistically significant. We were unable to conclude whether nocturnal hemodialysis yielded similar effects.
Trial registration: ClinicalTrials.gov NCT00264758 NCT00271999.
Copyright © 2013 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Treatment of depression and poor mental health among patients receiving maintenance dialysis: are there options other than a pill or a couch?Am J Kidney Dis. 2013 May;61(5):694-7. doi: 10.1053/j.ajkd.2013.02.347. Am J Kidney Dis. 2013. PMID: 23582252 Free PMC article. No abstract available.
References
-
- US Renal Data System. USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007.
-
- US Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2012;59:e1–e420. - PubMed
-
- Fukuhara S, Lopes AA, Bragg-Gresham JL, et al. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney international. 2003;64:1903–1910. - PubMed
-
- Kimmel PL, Cohen SD, Weisbord SD. Quality of life in patients with end-stage renal disease treated with hemodialysis: survival is not enough! Journal of nephrology. 2008;21(Suppl 13):S54–S58. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

