Angiotensin II drives the production of tumor-promoting macrophages
- PMID: 23333075
- PMCID: PMC3582771
- DOI: 10.1016/j.immuni.2012.10.015
Angiotensin II drives the production of tumor-promoting macrophages
Abstract
Macrophages frequently infiltrate tumors and can enhance cancer growth, yet the origins of the macrophage response are not well understood. Here we address molecular mechanisms of macrophage production in a conditional mouse model of lung adenocarcinoma. We report that overproduction of the peptide hormone Angiotensin II (AngII) in tumor-bearing mice amplifies self-renewing hematopoietic stem cells (HSCs) and macrophage progenitors. The process occurred in the spleen but not the bone marrow, and was independent of hemodynamic changes. The effects of AngII required direct hormone ligation on HSCs, depended on S1P(1) signaling, and allowed the extramedullary tissue to supply new tumor-associated macrophages throughout cancer progression. Conversely, blocking AngII production prevented cancer-induced HSC and macrophage progenitor amplification and thus restrained the macrophage response at its source. These findings indicate that AngII acts upstream of a potent macrophage amplification program and that tumors can remotely exploit the hormone's pathway to stimulate cancer-promoting immunity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

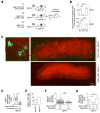

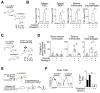
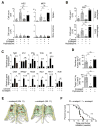
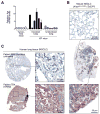
Comment in
-
Applying pressure on macrophages.Immunity. 2013 Feb 21;38(2):205-6. doi: 10.1016/j.immuni.2013.02.004. Immunity. 2013. PMID: 23438819
References
-
- AbdAlla S, Lother H, Langer A, el Faramawy Y, Quitterer U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell. 2004;119:343–354. - PubMed
-
- Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12:65–82. - PubMed
-
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. - PubMed
-
- Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan R, Iwamoto Y, Marinelli B, Gorbatov R, Forghani R, Novobrantseva TI, Koteliansky V, Figueiredo JL, Chen JW, Anderson DG, Nahrendorf M, Swirski FK, Weissleder R, Pittet M. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. - PMC - PubMed
-
- De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL095612/HL/NHLBI NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- P50 CA086355/CA/NCI NIH HHS/United States
- R01 NS070835/NS/NINDS NIH HHS/United States
- U54-CA126515/CA/NCI NIH HHS/United States
- R01-AI084880/AI/NIAID NIH HHS/United States
- P50-CA86355/CA/NCI NIH HHS/United States
- U54 CA126515/CA/NCI NIH HHS/United States
- R56 AI084880/AI/NIAID NIH HHS/United States
- R56-AI084880/AI/NIAID NIH HHS/United States
- R01 NS072167/NS/NINDS NIH HHS/United States
- R01 AI084880/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

