Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality-of-life outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) phase 3 randomised trial
- PMID: 23333117
- PMCID: PMC3596061
- DOI: 10.1016/S1470-2045(12)70567-3
Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality-of-life outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) phase 3 randomised trial
Abstract
Background: In the Gynecologic Cancer Intergroup International Collaboration on Ovarian Neoplasms 7 (ICON7) trial, bevacizumab improved progression-free survival in patients with ovarian cancer when used in combination with first-line chemotherapy and as a single-drug continuation treatment for 18 cycles. In a preliminary analysis of a high-risk subset of patients, there was also an improvement in overall survival. This study aims to describe the health-related quality-of-life (QoL) outcomes from ICON7.
Methods: ICON7 is a randomised, multicentre, open-label phase 3 trial. Between Dec 18, 2006, and Feb 16, 2009, after a surgical procedure aiming to debulk the disease, women with International Federation of Gynecology and Obstetrics (FIGO) high-risk stage I-IV epithelial ovarian cancer were randomly allocated (1:1) by computer program and block randomisation to receive either six cycles of standard chemotherapy (total 18 weeks) with carboplatin (area under the curve 5 or 6) and paclitaxel (175 mg/m(2)) alone or with bevacizumab (7·5 mg/kg) given intravenously with chemotherapy and continued as a single drug thereafter (total 54 weeks). The primary QoL endpoint was global QoL from the European Organisation for Research and Treatment of Cancer quality-of-life questionnaire-core 30 at week 54, analysed by ANOVA and adjusted for baseline score. Analyses were by intention to treat. The ICON7 trial has completed recruitment and remains in follow-up. This study is registered, number ISRCTN91273375.
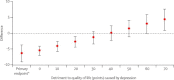
Findings: 764 women were randomly assigned to the standard chemotherapy group and 764 to the bevacizumab group. At baseline, 684 (90%) of women in the standard chemotherapy group and 691 (90%) of those in the bevacizumab group had completed QoL questionnaires. At week 54, 502 (66%) women in the bevacizumab group and 388 (51%) women in the standard chemotherapy group provided QoL data. Overall, the mean global QoL score improved during chemotherapy by 7·2 points (SD 24·4) when analysed for all women with data at baseline and week 18. The mean global QoL score at 54 weeks was higher in the standard chemotherapy group than in the bevacizumab group (76·1 [SD 18·2] vs 69·7 [19·1] points; difference 6·4 points, 95% CI 3·7-9·0, p<0·0001).
Interpretation: Bevacizumab continuation treatment seems to be associated with a small but clinically significant decrement in QoL compared with standard treatment for women with ovarian cancer. The trade-off between the prolongation of progression-free survival and the quality of that period of time needs to be considered in clinical practice when making treatment decisions.
Funding: Roche and the National Institute for Health Research through the UK National Cancer Research Network.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Quality of life in ICON7: need for patients' perspectives.Lancet Oncol. 2013 Mar;14(3):183-5. doi: 10.1016/S1470-2045(12)70590-9. Epub 2013 Jan 18. Lancet Oncol. 2013. PMID: 23333118 No abstract available.
-
Bevacizumab treatment and quality of life in advanced ovarian cancer.Future Oncol. 2013 Jul;9(7):951-4. doi: 10.2217/fon.13.77. Future Oncol. 2013. PMID: 23837758
References
-
- Yoneda J, Kuniyasu H, Crispens MA. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. - PubMed
-
- Huang S, Robinson JB, Deguzman A. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60:5334–5339. - PubMed
-
- Eskens FA, Sleijfer S. The use of bevacizumab in colorectal, lung, breast, renal and ovarian cancer: where does it fit? Eur J Cancer. 2008;44:2350–2356. - PubMed
-
- Perren TJ, Swart AM, Pfisterer J. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. - PubMed
-
- Bezjak A, Tu D, Bacon M. Quality of life in ovarian cancer patients: comparison of paclitaxel plus cisplatin, with cyclophosphamide plus cisplatin in a randomized study. J Clin Oncol. 2004;22:4595–4603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

