Helix bundle loops determine whether histidine kinases autophosphorylate in cis or in trans
- PMID: 23333741
- PMCID: PMC3636764
- DOI: 10.1016/j.jmb.2013.01.011
Helix bundle loops determine whether histidine kinases autophosphorylate in cis or in trans
Abstract
Bacteria frequently use two-component signal transduction pathways to sense and respond to environmental and intracellular stimuli. Upon receipt of a stimulus, a homodimeric sensor histidine kinase autophosphorylates and then transfers its phosphoryl group to a cognate response regulator. The autophosphorylation of histidine kinases has been reported to occur both in cis and in trans, but the molecular determinants dictating which mechanism is employed are unknown. Based on structural considerations, one model posits that the handedness of a loop at the base of the helical dimerization domain plays a critical role. Here, we tested this model by replacing the loop from Escherichia coli EnvZ, which autophosphorylates in trans, with the loop from three PhoR orthologs that autophosphorylate in cis. These chimeric kinases autophosphorylated in cis, indicating that this small loop is sufficient to determine autophosphorylation mechanism. Further, we report that the mechanism of autophosphorylation is conserved in orthologous sets of histidine kinases despite highly dissimilar loop sequences. These findings suggest that histidine kinases are under selective pressure to maintain their mode of autophosphorylation, but they can do so with a wide range of sequences.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
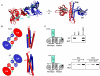




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

