IκB kinase ε (IKKε): a therapeutic target in inflammation and cancer
- PMID: 23333767
- PMCID: PMC7111187
- DOI: 10.1016/j.bcp.2013.01.007
IκB kinase ε (IKKε): a therapeutic target in inflammation and cancer
Abstract
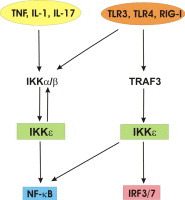
The innate immune system forms our first line of defense against invading pathogens and relies for a major part on the activation of two transcription factors, NF-κB and IRF3. Signaling pathways that activate these transcription factors are intertwined at the level of the canonical IκB kinases (IKKα, IKKβ) and non-canonical IKK-related kinases (IKKε, TBK1). Recently, significant progress has been made in understanding the function and mechanism of action of IKKε in immune signaling. In addition, IKKε impacts on cell proliferation and transformation, and is thereby also classified as an oncogene. Studies with IKKε knockout mice have illustrated a key role for IKKε in inflammatory and metabolic diseases. In this review we will highlight the mechanisms by which IKKε impacts on signaling pathways involved in disease development and discuss its potential as a novel therapeutic target.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Olive C. Pattern recognition receptors: sentinels in innate immunity and targets of new vaccine adjuvants. Expert Rev Vaccines. 2012;11:237–256. - PubMed
-
- Gilmore T.D., Wolenski F.S. NF-kappaB: where did it come from and why. Immunol Rev. 2012;246:14–35. - PubMed
-
- Peters R.T., Liao S.M., Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. - PubMed
-
- Tojima Y., Fujimoto A., Delhase M., Chen Y., Hatakeyama S., Nakayama K. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. - PubMed
-
- Chien Y., Kim S., Bumeister R., Loo Y.M., Kwon S.W., Johnson C.L. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

