High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria
- PMID: 23333862
- PMCID: PMC3595072
- DOI: 10.4161/gmic.23571
High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria
Abstract
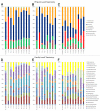
Fecal microbiota transplantation (FMT) is becoming a more widely used technology for treatment of recurrent Clostridum difficile infection (CDI). While previous treatments used fresh fecal slurries as a source of microbiota for FMT, we recently reported the successful use of standardized, partially purified and frozen fecal microbiota to treat CDI. Here we report that high-throughput 16S rRNA gene sequencing showed stable engraftment of gut microbiota following FMT using frozen fecal bacteria from a healthy donor. Similar bacterial taxa were found in post-transplantation samples obtained from the recipients and donor samples, but the relative abundance varied considerably between patients and time points. Post FMT samples from patients showed an increase in the abundance of Firmicutes and Bacteroidetes, representing 75-80% of the total sequence reads. Proteobacteria and Actinobacteria were less abundant (< 5%) than that found in patients prior to FMT. Post FMT samples from two patients were very similar to donor samples, with the Bacteroidetes phylum represented by a great abundance of members of the families Bacteroidaceae, Rikenellaceae and Porphyromonadaceae, and were largely comprised of Bacteroides, Alistipes and Parabacteroides genera. Members of the phylum Firmicutes were represented by Ruminococcaceae, Lachnospiraceae, Verrucomicrobiaceae and unclassified Clostridiales and members of the Firmicutes. One patient subsequently received antibiotics for an unrelated infection, resulting in an increase in the number of intestinal Proteobacteria, primarily Enterobacteriaceae. Our results demonstrate that frozen fecal microbiota from a healthy donor can be used to effectively treat recurrent CDI resulting in restoration of the structure of gut microbiota and clearing of Clostridum difficile.
Keywords: Clostridium difficile; DNA sequence analysis; Illumina; bacteroides; curing; fecal microbial transplantation; firmicutes; frozen preparations; microbiota; restoration.
Figures


References
-
- Kuijper EJ, Barbut F, Brazier JS, Kleinkauf N, Eckmanns T, Lambert ML, et al. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Euro Surveill. 2008;13 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
