Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence
- PMID: 23334421
- PMCID: PMC3561500
- DOI: 10.1038/nature11776
Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence
Abstract
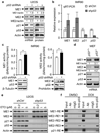
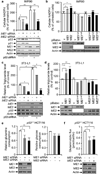
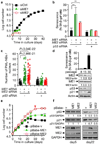
Cellular senescence both protects multicellular organisms from cancer and contributes to their ageing. The pre-eminent tumour suppressor p53 has an important role in the induction and maintenance of senescence, but how it carries out this function remains poorly understood. In addition, although increasing evidence supports the idea that metabolic changes underlie many cell-fate decisions and p53-mediated tumour suppression, few connections between metabolic enzymes and senescence have been established. Here we describe a new mechanism by which p53 links these functions. We show that p53 represses the expression of the tricarboxylic-acid-cycle-associated malic enzymes ME1 and ME2 in human and mouse cells. Both malic enzymes are important for NADPH production, lipogenesis and glutamine metabolism, but ME2 has a more profound effect. Through the inhibition of malic enzymes, p53 regulates cell metabolism and proliferation. Downregulation of ME1 and ME2 reciprocally activates p53 through distinct MDM2- and AMP-activated protein kinase-mediated mechanisms in a feed-forward manner, bolstering this pathway and enhancing p53 activation. Downregulation of ME1 and ME2 also modulates the outcome of p53 activation, leading to strong induction of senescence, but not apoptosis, whereas enforced expression of either malic enzyme suppresses senescence. Our findings define physiological functions of malic enzymes, demonstrate a positive-feedback mechanism that sustains p53 activation, and reveal a connection between metabolism and senescence mediated by p53.
Figures

References
-
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. - PubMed
-
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. - PubMed
-
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. - PubMed
-
- Hsu RY. Pigeon liver malic enzyme. Mol Cell Biochem. 1982;43:3–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

