Mutations in SLC20A2 are a major cause of familial idiopathic basal ganglia calcification
- PMID: 23334463
- PMCID: PMC4023541
- DOI: 10.1007/s10048-012-0349-2
Mutations in SLC20A2 are a major cause of familial idiopathic basal ganglia calcification
Abstract
Familial idiopathic basal ganglia calcification (IBGC) or Fahr's disease is a rare neurodegenerative disorder characterized by calcium deposits in the basal ganglia and other brain regions, which is associated with neuropsychiatric and motor symptoms. Familial IBGC is genetically heterogeneous and typically transmitted in an autosomal dominant fashion. We performed a mutational analysis of SLC20A2, the first gene found to cause IBGC, to assess its genetic contribution to familial IBGC. We recruited 218 subjects from 29 IBGC-affected families of varied ancestry and collected medical history, neurological exam, and head CT scans to characterize each patient's disease status. We screened our patient cohort for mutations in SLC20A2. Twelve novel (nonsense, deletions, missense, and splice site) potentially pathogenic variants, one synonymous variant, and one previously reported mutation were identified in 13 families. Variants predicted to be deleterious cosegregated with disease in five families. Three families showed nonsegregation with clinical disease of such variants, but retrospective review of clinical and neuroimaging data strongly suggested previous misclassification. Overall, mutations in SLC20A2 account for as many as 41% of our familial IBGC cases. Our screen in a large series expands the catalog of SLC20A2 mutations identified to date and demonstrates that mutations in SLC20A2 are a major cause of familial IBGC. Non-perfect segregation patterns of predicted deleterious variants highlight the challenges of phenotypic assessment in this condition with highly variable clinical presentation.
Conflict of interest statement
Figures
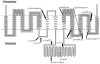
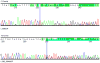

Comment in
-
Overall mutational spectrum of SLC20A2, PDGFB and PDGFRB in idiopathic basal ganglia calcification.Neurogenetics. 2014 Aug;15(3):215-6. doi: 10.1007/s10048-014-0404-2. Epub 2014 Apr 27. Neurogenetics. 2014. PMID: 24770784 No abstract available.
References
-
- Sobrido MJ, Hopfer S, Geschwind DH. Familial idiopathic basal ganglia calcification. In: Pagon R, Bird T, Dolan C, editors. GeneReviews [Internet] Seattle WA: 2007.
-
- Moskowitz M, Winickoff R, Heinz E. Familial calcification of the basal ganglions: a metabolic and genetic study. N Engl J Med. 1971;285(2):72–77. - PubMed
-
- Brodaty H, Mitchell P, Luscombe G, Kwok JJ, Badenhop RF, McKenzie R, et al. Familial idiopathic basal ganglia calcification (Fahr’s disease) without neurological, cognitive and psychiatric symptoms is not linked to the IBGC1 locus on chromosome 14q. Hum Genet. 2002;110(1):8–14. - PubMed
-
- Koller WC, Cochran JW, Klawans HL. Calcification of the basal ganglia: computerized tomography and clinical correlation. Neurology. 1979;29(3):328–333. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

