Friedreich ataxia: neuropathology revised
- PMID: 23334592
- PMCID: PMC3817014
- DOI: 10.1097/NEN.0b013e31827e5762
Friedreich ataxia: neuropathology revised
Abstract
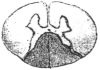
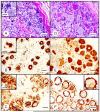
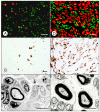
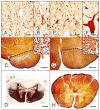
Friedreich ataxia is an autosomal recessive disorder that affects children and young adults. The mutation consists of a homozygous guanine-adenine-adenine trinucleotide repeat expansion that causes deficiency of frataxin, a small nuclear genome-encoded mitochondrial protein. Low frataxin levels lead to insufficient biosynthesis of iron-sulfur clusters that are required for mitochondrial electron transport and assembly of functional aconitase, and iron dysmetabolism of the entire cell. This review of the neuropathology of Friedreich ataxia stresses the critical role of hypoplasia and superimposed atrophy of dorsal root ganglia. Progressive destruction of dorsal root ganglia accounts for thinning of dorsal roots, degeneration of dorsal columns, transsynaptic atrophy of nerve cells in Clarke column and dorsal spinocerebellar fibers, atrophy of gracile and cuneate nuclei, and neuropathy of sensory nerves. The lesion of the dentate nucleus consists of progressive and selective atrophy of large glutamatergic neurons and grumose degeneration of corticonuclear synaptic terminals that contain γ-aminobutyric acid (GABA). Small GABA-ergic neurons and their projection fibers in the dentato-olivary tract survive. Atrophy of Betz cells and corticospinal tracts constitute a second intrinsic CNS lesion. In light of the selective vulnerability of organs and tissues to systemic frataxin deficiency, many questions about the pathogenesis of Friedreich ataxia remain.
Figures



References
-
- Friedreich N. Ueber Ataxie mit besonderer Berücksichtigung der hereditären Formen. Nachtrag. Virchows Arch Pathol Anat Physiol Klin Med. 1877;70:140–52.
-
- Koeppen AH, Morral JA, Davis AN, et al. The dorsal root ganglion in Friedreich’s ataxia. Acta Neuropathol. 2009;118:763–76. - PubMed
-
- Morral JA, Davis AN, Qian J, et al. Pathology and pathogenesis of sensory neuropathy in Friedreich’s ataxia. Acta Neuropathol (Berl) 2010;120:97–108. - PubMed
-
- Mott FW. Case of Friedreich’s disease, with autopsy and systematic microscopical examination of the nervous system. Arch Neurol Psychiat (Lond) 1907;3:180–200.
-
- Spiller WG. Friedreich’s ataxia. J Nerv Ment Dis. 1910;37:411–35.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

