Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations
- PMID: 23334667
- PMCID: PMC3739288
- DOI: 10.1038/ng.2526
Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations
Abstract
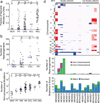
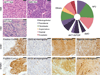
Meningiomas are the most common primary nervous system tumor. The tumor suppressor NF2 is disrupted in approximately half of all meningiomas, but the complete spectrum of genetic changes remains undefined. We performed whole-genome or whole-exome sequencing on 17 meningiomas and focused sequencing on an additional 48 tumors to identify and validate somatic genetic alterations. Most meningiomas had simple genomes, with fewer mutations, rearrangements and copy-number alterations than reported in other tumors in adults. However, several meningiomas harbored more complex patterns of copy-number changes and rearrangements, including one tumor with chromothripsis. We confirmed focal NF2 inactivation in 43% of tumors and found alterations in epigenetic modifiers in an additional 8% of tumors. A subset of meningiomas lacking NF2 alterations harbored recurrent oncogenic mutations in AKT1 (p.Glu17Lys) and SMO (p.Trp535Leu) and exhibited immunohistochemical evidence of activation of these pathways. These mutations were present in therapeutically challenging tumors of the skull base and higher grade. These results begin to define the spectrum of genetic alterations in meningiomas and identify potential therapeutic targets.
Figures


Comment in
-
Neuro-oncology: new therapeutic targets identified in meningiomas.Nat Rev Neurol. 2013 Mar;9(3):121. doi: 10.1038/nrneurol.2013.16. Epub 2013 Feb 12. Nat Rev Neurol. 2013. PMID: 23399644 No abstract available.
-
SMO and AKT1 mutations occur in non-NF2 meningiomas.Cancer Discov. 2013 Mar;3(3):OF13. doi: 10.1158/2159-8290.CD-RW2013-028. Epub 2013 Feb 7. Cancer Discov. 2013. PMID: 23475883
References
-
- Choy W, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30:E6. - PubMed
-
- Durand A, et al. WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol. 2009;95:367–375. - PubMed
-
- Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. "Malignancy" in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046–2056. - PubMed
-
- Goutagny S, et al. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin Cancer Res. 2010;16:4155–4164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

