Transcriptional reprogramming of mature CD4⁺ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes
- PMID: 23334788
- PMCID: PMC3581083
- DOI: 10.1038/ni.2523
Transcriptional reprogramming of mature CD4⁺ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes
Abstract
TCRαβ thymocytes differentiate into either CD8αβ(+) cytotoxic T lymphocytes or CD4(+) helper T cells. This functional dichotomy is controlled by key transcription factors, including the helper T cell master regulator ThPOK, which suppresses the cytolytic program in major histocompatibility complex (MHC) class II-restricted CD4(+) thymocytes. ThPOK continues to repress genes of the CD8 lineage in mature CD4(+) T cells, even as they differentiate into effector helper T cell subsets. Here we found that the helper T cell fate was not fixed and that mature, antigen-stimulated CD4(+) T cells terminated expression of the gene encoding ThPOK and reactivated genes of the CD8 lineage. This unexpected plasticity resulted in the post-thymic termination of the helper T cell program and the functional differentiation of distinct MHC class II-restricted CD4(+) cytotoxic T lymphocytes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



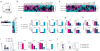
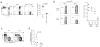
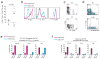
Comment in
-
T cells: The plastic virtues of a CD4+ T cell.Nat Rev Immunol. 2013 Mar;13(3):151. doi: 10.1038/nri3400. Epub 2013 Feb 1. Nat Rev Immunol. 2013. PMID: 23370307 No abstract available.
-
Changing course by lymphocyte lineage redirection.Nat Immunol. 2013 Mar;14(3):199-201. doi: 10.1038/ni.2544. Nat Immunol. 2013. PMID: 23416670 No abstract available.
References
-
- Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. - PubMed
-
- Pai SY, et al. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. - PubMed
-
- He X, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. - PubMed
-
- Sun G, et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

