Dysmaturation of the default mode network in autism
- PMID: 23334984
- PMCID: PMC3651798
- DOI: 10.1002/hbm.22252
Dysmaturation of the default mode network in autism
Abstract
Two hypotheses of autism spectrum disorder (ASD) propose that this condition is characterized by deficits in Theory of Mind and by hypoconnectivity between remote cortical regions with hyperconnectivity locally. The default mode network (DMN) is a set of remote, functionally connected cortical nodes less active during executive tasks than at rest and is implicated in Theory of Mind, episodic memory, and other self-reflective processes. We show that children with ASD have reduced connectivity between DMN nodes and increased local connectivity within DMN nodes and the visual and motor resting-state networks. We show that, like the trajectory of synaptogenesis, internodal DMN functional connectivity increased as a quadratic function of age in typically developing children, peaking between, 11 and 13 years. In children with ASD, these long-distance connections fail to develop during adolescence. These findings support the "developmental disconnection model" of ASD, provide a possible mechanistic explanation for the Theory-of-Mind hypothesis of ASD, and show that the window for effectively treating ASD could be wider than previously thought.
Keywords: Theory of Mind; autism; default mode network; development; functional connectivity; synaptogenesis.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
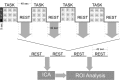
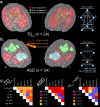
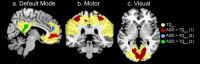
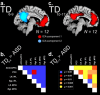


References
-
- Baron‐Cohen S (1989): The theory of mind hypothesis of autism: A reply to Boucher. Br J Disord Commun 24:199–200. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

