Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1α-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin
- PMID: 23335089
- PMCID: PMC3755376
- DOI: 10.1002/pros.22644
Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1α-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin
Abstract
Background: Prostate cancer is the most common malignancy and second leading cause of cancer related deaths in American men supporting the study of prostate cancer chemoprevention. Major risk factors for this disease have been associated with low serum levels of vitamin D. Here, we evaluate the biologic activity of a less calcemic vitamin D analog 1α-hydroxyvitamin D2 [1α-OH-D2] (Bone Care International, Inc.) in patients with prostate cancer and high grade prostatic intraepithelial neoplasia (HG PIN).
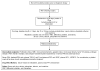
Methods: Patients with clinically organ-confined prostate cancer and HG PIN were randomized to 1α-OH-D2 versus placebo for 28 days prior to radical prostatectomy. Intermediate endpoint biomarkers included serum vitamin D metabolites, TGFß 1/2, free/total PSA, IGF-1, IGFBP-3, bFGF, and VEGF. Tissue endpoints included histology, MIB-1 and TUNEL staining, microvessel density and factor VIII staining, androgen receptor and PSA, vitamin D receptor expression and nuclear morphometry.
Results: The 1α-OH-D2 vitamin D analog was well tolerated and could be safely administered with good compliance and no evidence of hypercalcemia over 28 days. While serum vitamin D metabolite levels only slightly increased, evidence of biologic activity was observed with significant reductions in serum PTH levels. TGF-ß2 was the only biomarker significantly altered by vitamin D supplementation. Whether reduced TGF-ß2 levels in our study is an early indicator of response to vitamin D remains unclear.
Conclusions: While further investigation of vitamin D may be warranted based on preclinical studies, results of the present trial do not appear to justify evaluation of 1α-OH-D2 in larger clinical prostate cancer prevention studies.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

Similar articles
-
A phase II randomized placebo-controlled trial of pomegranate fruit extract in men with localized prostate cancer undergoing active surveillance.Prostate. 2021 Jan;81(1):41-49. doi: 10.1002/pros.24076. Epub 2020 Oct 23. Prostate. 2021. PMID: 33095939 Clinical Trial.
-
Interactions of the Insulin-Like Growth Factor Axis and Vitamin D in Prostate Cancer Risk in the Prostate Cancer Prevention Trial.Nutrients. 2017 Apr 12;9(4):378. doi: 10.3390/nu9040378. Nutrients. 2017. PMID: 28417914 Free PMC article. Clinical Trial.
-
Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months.Br J Nutr. 2013 Mar 28;109(6):1082-8. doi: 10.1017/S0007114512002851. Epub 2012 Jul 11. Br J Nutr. 2013. PMID: 23168298 Clinical Trial.
-
Prostatic intraepithelial neoplasia (PIN) and other prostatic lesions as risk factors and surrogate endpoints for cancer chemoprevention trials.J Cell Biochem Suppl. 1996;25:156-64. J Cell Biochem Suppl. 1996. PMID: 9027613 Review.
-
The role of vitamin D in prostate cancer.Steroids. 2001 Mar-May;66(3-5):293-300. doi: 10.1016/s0039-128x(00)00164-1. Steroids. 2001. PMID: 11179737 Review.
Cited by
-
Randomized, Placebo-Controlled Trial of Green Tea Catechins for Prostate Cancer Prevention.Cancer Prev Res (Phila). 2015 Oct;8(10):879-87. doi: 10.1158/1940-6207.CAPR-14-0324. Epub 2015 Apr 14. Cancer Prev Res (Phila). 2015. PMID: 25873370 Free PMC article. Clinical Trial.
-
An integrated approach to defining genetic and environmental determinants for major clinical outcomes involving vitamin D.Mol Diagn Ther. 2014 Jun;18(3):261-72. doi: 10.1007/s40291-014-0087-2. Mol Diagn Ther. 2014. PMID: 24557774 Free PMC article. Review.
-
Prostate cancer chemoprevention by natural agents: Clinical evidence and potential implications.Cancer Lett. 2018 May 28;422:9-18. doi: 10.1016/j.canlet.2018.02.025. Epub 2018 Feb 20. Cancer Lett. 2018. PMID: 29471004 Free PMC article. Review.
-
Reassessing the Association between Circulating Vitamin D and IGFBP-3: Observational and Mendelian Randomization Estimates from Independent Sources.Cancer Epidemiol Biomarkers Prev. 2018 Dec;27(12):1462-1471. doi: 10.1158/1055-9965.EPI-18-0113. Epub 2018 Aug 2. Cancer Epidemiol Biomarkers Prev. 2018. PMID: 30072546 Free PMC article.
-
Dutasteride for the prevention of prostate cancer in men with high-grade prostatic intraepithelial neoplasia: results of a phase III randomized open-label 3-year trial.World J Urol. 2017 May;35(5):721-728. doi: 10.1007/s00345-016-1938-8. Epub 2016 Sep 19. World J Urol. 2017. PMID: 27644229 Clinical Trial.
References
-
- Siegel R, Naishadham MA, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Brawley OW, Barnes S, Parnes H. The future of prostate cancer prevention. Ann NY Acad Sci. 2001;952:145–152. - PubMed
-
- Davidson D, Bostwick DG, Qian J, Wollan PC, Oesterling JE, Rudders RA, Siroky M, Stilmant M. Prostatic intraepithelial neoplasia is a risk factor for adenocarcinoma: Predictive accuracy in needle biopsies. J Urol. 1995;154:1295–1299. - PubMed
-
- Ahn J, Moslehi R, Weinstein SJ, Snyder K, Virtamo J, Albanes D. Family history of prostate cancer and prostate cancer risk in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. Int J Cancer. 2008;123(5):1154–1159. - PubMed
-
- Lieberman R. Prostate cancer chemoprevention: Strategies for designing efficient clinical trials. Urology. 2001;57(4 Suppl 1):224–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

