Fowlpox-based survivin vaccination for malignant mesothelioma therapy
- PMID: 23335100
- PMCID: PMC3663911
- DOI: 10.1002/ijc.28048
Fowlpox-based survivin vaccination for malignant mesothelioma therapy
Abstract
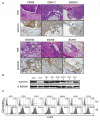
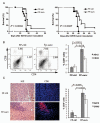
Survivin protein is an attractive candidate for cancer immunotherapy since it is abundantly expressed in most common human cancers and mostly absent in normal adult tissues. Malignant mesothelioma (MM) is a deadly cancer associated with asbestos or erionite exposure for which no successful therapies are currently available. In this study, we evaluated the therapeutic efficacy of a novel survivin-based vaccine by subcutaneous or intraperitoneum injection of BALB/c mice with murine fiber-induced MM tumor cells followed by vaccination with recombinant Fowlpox virus replicons encoding survivin. Vaccination generated significant immune responses in both models, leading to delayed tumor growth and improved animal survival. Flow cytometry and immunofluorescence analyses of tumors from vaccinated mice showed CD8(+) T-cell infiltration, and real-time PCR demonstrated increased mRNA and protein levels of immunostimulatory cytokines. Analyses of survivin peptide-pulsed spleen and lymph node cells from vaccinated mice using ELISPOT and intracellular cytokine staining confirmed antigen-specific, interferon-γ-producing CD8(+) T-cell responses. In addition pentamer-based flow cytometry showed that vaccination generated survivin-specific CD8(+) T cells. Importantly, vaccination did not affect fertility or induce autoimmune abnormalities in mice. Our results demonstrate that vaccination with recombinant Fowlpox expressing survivin improves T-cell responses against aggressive MM tumors and may form the basis for promising clinical applications.
Copyright © 2013 UICC.
Figures



Similar articles
-
Preclinical development of HIvax: Human survivin highly immunogenic vaccines.Hum Vaccin Immunother. 2015;11(7):1585-95. doi: 10.1080/21645515.2015.1050572. Hum Vaccin Immunother. 2015. PMID: 26042612 Free PMC article.
-
Prime-boost vaccination with SA-4-1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner.PLoS One. 2012;7(11):e48463. doi: 10.1371/journal.pone.0048463. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23144888 Free PMC article.
-
Modified vaccinia Ankara expressing survivin combined with gemcitabine generates specific antitumor effects in a murine pancreatic carcinoma model.Cancer Immunol Immunother. 2011 Jan;60(1):99-109. doi: 10.1007/s00262-010-0923-0. Epub 2010 Oct 20. Cancer Immunol Immunother. 2011. PMID: 20960189 Free PMC article.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
Novel targeted therapies and vaccination strategies for mesothelioma.Curr Treat Options Oncol. 2011 Jun;12(2):149-62. doi: 10.1007/s11864-011-0149-1. Curr Treat Options Oncol. 2011. PMID: 21424750 Review.
Cited by
-
Utility of Survivin, BAP1, and Ki-67 immunohistochemistry in distinguishing epithelioid mesothelioma from reactive mesothelial hyperplasia.Oncol Lett. 2018 Mar;15(3):3540-3547. doi: 10.3892/ol.2018.7765. Epub 2018 Jan 10. Oncol Lett. 2018. PMID: 29467873 Free PMC article.
-
Increasing dietary selenium elevates reducing capacity and ERK activation associated with accelerated progression of select mesothelioma tumors.Am J Pathol. 2014 Apr;184(4):1041-1049. doi: 10.1016/j.ajpath.2013.12.008. Epub 2014 Feb 1. Am J Pathol. 2014. PMID: 24492200 Free PMC article.
-
Preclinical development of HIvax: Human survivin highly immunogenic vaccines.Hum Vaccin Immunother. 2015;11(7):1585-95. doi: 10.1080/21645515.2015.1050572. Hum Vaccin Immunother. 2015. PMID: 26042612 Free PMC article.
-
Vaccination with a piggyBac plasmid with transgene integration potential leads to sustained antigen expression and CD8(+) T cell responses.Vaccine. 2014 Mar 26;32(15):1670-7. doi: 10.1016/j.vaccine.2014.01.063. Epub 2014 Feb 7. Vaccine. 2014. PMID: 24513010 Free PMC article.
-
Latest developments in our understanding of the pathogenesis of mesothelioma and the design of targeted therapies.Expert Rev Respir Med. 2015 Oct;9(5):633-54. doi: 10.1586/17476348.2015.1081066. Epub 2015 Aug 26. Expert Rev Respir Med. 2015. PMID: 26308799 Free PMC article. Review.
References
-
- Bertino P, Carbone M, Pass H. Chemotherapy of malignant pleural mesothelioma. Expert opinion on pharmacotherapy. 2009;10:99–107. - PubMed
-
- Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, Johnston MR, de Perrot M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. The Journal of thoracic and cardiovascular surgery. 2008;135:823–9. - PubMed
-
- Yamada N, Oizumi S, Kikuchi E, Shinagawa N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T, Torigoe T, Nishimura M. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer immunology, immunotherapy : CII. 2010;59:1543–9. - PMC - PubMed
-
- Lladser A, Sanhueza C, Kiessling R, Quest AF. Is survivin the potential Achilles' heel of cancer? Advances in cancer research. 2011;111:1–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G12 MD007601/MD/NIMHD NIH HHS/United States
- R01 AI089999/AI/NIAID NIH HHS/United States
- R01AI089999/AI/NIAID NIH HHS/United States
- P01 CA114047/CA/NCI NIH HHS/United States
- P01CA114047/CA/NCI NIH HHS/United States
- P20GM103516/GM/NIGMS NIH HHS/United States
- R01 CA160715/CA/NCI NIH HHS/United States
- R01CA160715/CA/NCI NIH HHS/United States
- G12RR003061/RR/NCRR NIH HHS/United States
- R21 AT004844/AT/NCCIH NIH HHS/United States
- G12 RR003061/RR/NCRR NIH HHS/United States
- G12MD007601/MD/NIMHD NIH HHS/United States
- P20 GM103466/GM/NIGMS NIH HHS/United States
- P30 CA071789/CA/NCI NIH HHS/United States
- P20 GM103516/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

