Etravirine in CSF is highly protein bound
- PMID: 23335197
- PMCID: PMC3625433
- DOI: 10.1093/jac/dks517
Etravirine in CSF is highly protein bound
Abstract
Objectives: Etravirine has high affinity for plasma drug-binding proteins, such as albumin and α1-acid glycoprotein, which limits the amount of unbound etravirine available to enter the CNS. The objective of this study was to compare total and unbound etravirine concentrations in CSF with plasma concentrations and the in vitro median inhibitory concentration (IC50) for wild-type HIV (0.9 ng/mL).
Methods: Total and bound etravirine concentrations were measured in 17 CSF and plasma pairs by isotope-dilution liquid chromatography tandem mass spectroscopy, radioligand displacement and ultracentrifugation. Unbound etravirine concentrations were calculated from the bound fraction. The dynamic range of the assay was 7.8-2000 (plasma) and 0.78-200 (CSF) ng/mL.
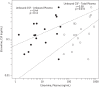
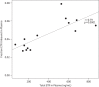
Results: Subjects were mostly middle-aged (median 43 years) white (78%) men (89%). All CSF etravirine concentrations were above the limit of quantification. Total and unbound median etravirine concentrations in CSF were 9.5 (IQR 6.4, 26.4) and 0.13 (IQR 0.08, 0.27) ng/mL, respectively. Etravirine was 96% (IQR 94.5, 97.2) protein bound in plasma and 98.4% (IQR 97.8, 98.8) in CSF. Total etravirine in CSF was 4.3% (IQR 3, 5.9) of total and 101% (IQR 76, 160) of unbound etravirine in plasma. There were no significant correlations between unbound etravirine concentrations and concentrations of albumin in plasma or CSF. Unbound etravirine concentrations in CSF did not reach the wild-type IC50 in any of the specimens.
Conclusions: Unbound etravirine may not achieve optimal concentrations to inhibit HIV replication in the CNS.
Figures

References
-
- Letendre SL, van den Brande G, Hermes A, et al. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin Infect Dis. 2007;45:1511–7.
-
- Katlama C, Clotet B, Mills A, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther. 2010;15:1045–52. - PubMed
-
- Tibotec Therapeutics. Intelence (Etravirine) Tablets: Full Prescribing Information. Titusville, NJ: Tibotec Therapeutics; 2008.
Publication types
MeSH terms
Substances
Grants and funding
- U54 HD071600-01/HD/NICHD NIH HHS/United States
- R01 MH58076/MH/NIMH NIH HHS/United States
- DA022137/DA/NIDA NIH HHS/United States
- R01 MH83552/MH/NIMH NIH HHS/United States
- U13 MH81676/MH/NIMH NIH HHS/United States
- P30 MH62512/MH/NIMH NIH HHS/United States
- MH22005/MH/NIMH NIH HHS/United States
- MH058076/MH/NIMH NIH HHS/United States
- P50 DA26306/DA/NIDA NIH HHS/United States
- U01 NS45911-04/NS/NINDS NIH HHS/United States
- P01 DA026146/DA/NIDA NIH HHS/United States
- 1R01NS074409-01A1/NS/NINDS NIH HHS/United States
- R01 MH78748/MH/NIMH NIH HHS/United States
- U01 AI 68632/AI/NIAID NIH HHS/United States
- N01MH22005/MH/NIMH NIH HHS/United States
- MH79886/MH/NIMH NIH HHS/United States
- R01MH83552/MH/NIMH NIH HHS/United States
- U01 AI69432/AI/NIAID NIH HHS/United States
- R01MH058076/MH/NIMH NIH HHS/United States
- K30RR22681/RR/NCRR NIH HHS/United States
- R25MH080663/MH/NIMH NIH HHS/United States
- U01MH083501/MH/NIMH NIH HHS/United States
- R01MH083627/MH/NIMH NIH HHS/United States
- 2U01NS32228/NS/NINDS NIH HHS/United States
- U01 MH83506/MH/NIMH NIH HHS/United States
- U01MH83506/MH/NIMH NIH HHS/United States
- NS32228/NS/NINDS NIH HHS/United States
- N01 MH22005/MH/NIMH NIH HHS/United States
- NS072005/NS/NINDS NIH HHS/United States
- P01 DA12065/DA/NIDA NIH HHS/United States
- U2G PS00623/PS/NCHHSTP CDC HHS/United States
- P30MH62512/MH/NIMH NIH HHS/United States
- R01 MH92225/MH/NIMH NIH HHS/United States
- R01 AG15301/AG/NIA NIH HHS/United States
- P50DA26306/DA/NIDA NIH HHS/United States
- R01MH095621/MH/NIMH NIH HHS/United States
- AI69495/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

