A novel interaction between aging and ER overload in a protein conformational dementia
- PMID: 23335331
- PMCID: PMC3584003
- DOI: 10.1534/genetics.112.149088
A novel interaction between aging and ER overload in a protein conformational dementia
Abstract
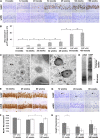
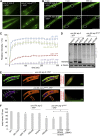
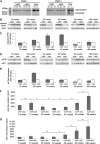
Intraneuronal deposition of aggregated proteins in tauopathies, Parkinson disease, or familial encephalopathy with neuroserpin inclusion bodies (FENIB) leads to impaired protein homeostasis (proteostasis). FENIB represents a conformational dementia, caused by intraneuronal polymerization of mutant variants of the serine protease inhibitor neuroserpin. In contrast to the aggregation process, the kinetic relationship between neuronal proteostasis and aggregation are poorly understood. To address aggregate formation dynamics, we studied FENIB in Caenorhabditis elegans and mice. Point mutations causing FENIB also result in aggregation of the neuroserpin homolog SRP-2 most likely within the ER lumen in worms, recapitulating morphological and biochemical features of the human disease. Intriguingly, we identified conserved protein quality control pathways to modulate protein aggregation both in worms and mice. Specifically, downregulation of the unfolded protein response (UPR) pathways in the worm favors mutant SRP-2 accumulation, while mice overexpressing a polymerizing mutant of neuroserpin undergo transient induction of the UPR in young but not in aged mice. Thus, we find that perturbations of proteostasis through impairment of the heat shock response or altered UPR signaling enhance neuroserpin accumulation in vivo. Moreover, accumulation of neuroserpin polymers in mice is associated with an age-related induction of the UPR suggesting a novel interaction between aging and ER overload. These data suggest that targets aimed at increasing UPR capacity in neurons are valuable tools for therapeutic intervention.
Figures



References
-
- Balch W. E., Morimoto R. I., Dillin A., Kelly J. W., 2008. Adapting proteostasis for disease intervention. Science 319: 916–919. - PubMed
-
- Borges V. M., Lee T. W., Christie D. L., Birch N. P., 2010. Neuroserpin regulates the density of dendritic protrusions and dendritic spine shape in cultured hippocampal neurons. J. Neurosci. Res. 88: 2610–2617. - PubMed
-
- Brandt R., Gergou A., Wacker I., Fath T., Hutter H., 2009. A Caenorhabditis elegans model of tau hyperphosphorylation: induction of developmental defects by transgenic overexpression of Alzheimer’s disease-like modified tau. Neurobiol. Aging 30: 22–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

